Abstract
Background
Epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors are the standard first-line treatment for patients with advanced and recurrent EGFR-positive non-small cell lung cancer.
Objective
The main objective of the present study was to compare the clinical efficacies between osimertinib and afatinib as first-line treatment in patients with EGFR-mutant non-small cell lung cancer.
Methods
We retrospectively analyzed patients with advanced and recurrent non-small cell lung cancer who harbored an exon 19 deletion or an exon 21 L858R mutation and were being given either osimertinib or afatinib as first-line treatment from January 2018 to December 2020.
Results
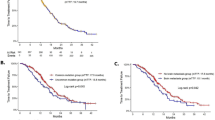
A total of 128 patients were selected for this study. The osimertinib group included 47 patients, while 81 patients received afatinib. The median follow-up time was 20.1 months in the osimertinib group and 22.7 months in the afatinib group. The median progression-free survival was 18.8 months and 13.1 months in the osimertinib and afatinib groups, respectively (hazard ratio 0.75 [95% confidence interval 0.48–1.18]). The median overall survival was not reached in the osimertinib group and was 41.7 months in the afatinib group (hazard ratio 0.79 [95% confidence interval 0.36–1.72]). In patients without brain metastasis, the median progression-free survival was 17.9 months and 17.2 months in the osimertinib and afatinib groups, respectively (hazard ratio 1.02 [95% confidence interval 0.56–1.85]). In patients with brain metastasis at baseline, the median progression-free survival was 22.1 months in the osimertinib group, and 10.9 months in the afatinib group (adjusted hazard ratio 0.45 [95% confidence interval 0.21–0.96]).
Conclusions
Our research demonstrates that there was no strong evidence showing that patients taking osimertinib as first-line treatment experienced longer median progression-free survival and overall survival than patients treated with afatinib. However, there was a statistical significance revealing that osimertinib provided better median progression-free survival than afatinib in patients with brain metastasis at baseline.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Patients with advanced epidermal growth factor receptor mutant non-small cell lung cancer undergoing osimertinib treatment did not experience significantly longer median progression-free survival and overall survival than those being treated with afatinib. |
In patients with non-small cell lung cancer with brain metastasis, patients receiving osimertinib experienced statistically better median progression-free survival than those taking afatinib. |
There was no difference regarding median progression-free survival in patients without brain metastasis between the osimertinib and afatinib groups. |
1 Introduction
Currently, the treatment strategy for patients with advanced non-small cell lung cancer (NSCLC) is personalized and depends on the results of molecular biological tests. Previous studies have shown that patients with lung adenocarcinoma with an actionable oncogenic driver mutation who are receiving matched targeted therapy will experience longer overall survival (OS) [1]. In Asia, approximately 50–60% of patients with lung adenocarcinoma harbor epidermal growth factor receptor (EGFR) mutations [2, 3]. Fortunately, previous clinical trials have demonstrated that patients with advanced NSCLC with a sensitizing EGFR mutation who were undergoing first-generation and second-generation EGFR-tyrosine kinase inhibitor (TKI) treatment experienced better progression-free survival (PFS), while exhibiting fewer adverse effects than those patients receiving platinum-based chemotherapy [4,5,6]. At present, EGFR-TKIs are the standard first-line treatment for patients with advanced EGFR-positive NSCLC.
Recently, certain clinical trials have been conducted to compare the clinical efficacies between different generation EGFR-TKIs. The LUX-Lung 7 study, a phase IIB trial, demonstrated that patients with advanced EGFR-mutant NSCLC receiving afatinib, a second-generation EGFR-TKI as first-line treatment, experienced longer median PFS than those receiving gefitinib, a first-generation EGFR-TKI [7]. The ARCHER-1050 study, a phase III trial, showed that dacomitinib, a second-generation EGFR-TKI, provided longer median PFS and OS than gefitinib in patients with treatment-naive EGFR-mutated NSCLC without the occurrence of any central nervous system metastasis [8, 9]. Real-world retrospective studies have also confirmed the results taken from clinical trials that showed that patients with advanced or recurrent EGFR-mutant NSCLC receiving second-generation EGFR-TKIs experienced better median PFS than those treated with first-generation EGFR-TKIs [10,11,12]. Furthermore, the FLAURA study proved that patients with treatment-naïve advanced NSCLC with EGFR mutations receiving the third-generation EGFR-TKI osimertinib experienced significantly better median PFS and OS than those treated with gefitinib and erlotinib [13, 14].
The FLAURA study provides truly promising data for treatment with first-line EGFR-TKIs. However, the GioTag study also showed a good median time on treatment and OS in patients with EGFR-mutant NSCLC undergoing sequential afatinib and osimertinib treatment [15]. Therefore, the difference in the clinical outcomes between second-generation and third-generation EGFR-TKIs for first-line treatment has become an important issue. Although clinical trials for comparing the efficacy of second-generation and third-generation EGFR-TKIs are ongoing (NCT04413201, jRCTs031190221), some retrospective studies have been published that demonstrate that there was no significant difference in either median PFS or time to discontinuation of TKI treatment between afatinib and osimertinib [16, 17]. However, the median follow-up time was not equal between the two groups in these studies, and the study population was relatively small in one of the two studies. Thus, we conducted the present study in order to compare the clinical outcomes between osimertinib and afatinib for patients with advanced and recurrent NSCLC with EGFR mutation within the Taiwanese population.
2 Material and Methods
2.1 Study Design and Patients
This study was a retrospective, single-center, observational study performed at Taichung Veterans General Hospital in Taiwan. The study was approved by the Institutional Review Board of Taichung Veterans General Hospital, Taiwan, with written informed consent documents for genetic testing and clinical data records obtained from all patients (No. CF12019).
Patients diagnosed with lung cancer between January 2018 and December 2020 were included in the study. All patients were required to fulfill the following inclusion criteria: a diagnosis of histologically and cytologically confirmed NSCLC, recurrence or stage IV lung cancer according to the 8th edition of the American Joint Committee for Cancer staging system [18], harboring a EGFR exon 19 deletion or exon 21 L858R point mutation, and receiving first-line treatment involving either osimertinib or afatinib. Patients were excluded if they had EGFR mutations other than the exon 19 deletion or the exon 21 L858R point mutation, were taking EGFR-TKIs other than osimertinib and afatinib, or had undergone combined treatment with either chemotherapy or anti-angiogenesis agents. Computed tomography of the chest of each patient was performed every 3 months in order to qualify for National Health Insurance reimbursement. Treatment response to osimertinib and afatinib was evaluated through the Response Evaluation Criteria in Solid Tumors (Version 1.1) [19].
Each patient’s demographic and clinical data, including age, sex, smoking status, Eastern Cooperative Oncology Group Performance Status (ECOG PS), clinical stage, status of brain metastasis at baseline, subtype of EGFR mutation at baseline, best response to EGFR-TKIs, and the PFS and OS of osimertinib and afatinib, were all recorded for analysis. We defined PFS as the time period from the first dose of EGFR-TKI to either progression or death, while the definition of OS was determined as the time period from the first dose of EGFR-TKI to death.
2.2 EGFR Mutation Test
EGFR mutation status in tumor tissue was tested by either the cobas® EGFR Mutation Test version 2 (Roche Molecular Systems, Pleasanton, CA, USA) or matrix-assisted laser desorption ionization-time of flight mass spectrometry. The method for matrix-assisted laser desorption ionization-time of flight mass spectrometry was based upon our previous studies [3, 20,21,22]. The detection procedure was performed according to the user’s manual of the MassARRAY® System (Cat. No. 10411, SEQUENIM, San Diego, CA, USA acquired by Agena Bioscience, http://agenabio.com/, San Diego, CA, USA in 2014). Extracted DNA was used to perform serial biochemical reactions, including 40 cycles of PCR; SAP (Shrimp Alkaline Phosphatase) treatment and 200 cycles of signal nucleotide extension reaction by using the iPLEX Pro® reagent kit containing Sequenase, iPLEX Pro® reaction mixture, and home-designed probes. After the SpectroClean Resin clean-up, samples were loaded onto the matrix of the SpectroCHIP by Nanodispenser (Matrix) and then analyzed using the Bruker Autoflex matrix-assisted laser desorption ionization-time of flight mass spectrometry. Data were collected and analyzed by MassARRAYTyper (Version 4) software (Agena Bioscience).
2.3 Statistical Analyses
We used the Fisher’s exact test to assess the differences in patient characteristics and demographic data between the osimertinib and afatinib groups. Survival curves for PFS and OS were estimated through the Kaplan–Meier method. The Cox proportional hazard model was used to evaluate the univariate and multivariate analyses for survival times of PFS and OS. All statistical tests were done with SPSS 23.0 (SPSS Inc., Chicago, IL, USA) software. Two-tailed tests and p values < 0.05 for significance were used.
3 Results
3.1 Clinical and Demographic Baseline Characteristics of Patients Treated with Osimertinib or Afatinib as First-Line Treatment
A total of patients with 128 stage IV and recurrent NSCLC who had been treated with either osimertinib or afatinib as first-line treatment were included in our analysis. The osimertinib group included 47 patients, while 81 patients received afatinib. The flowchart of patient collection is demonstrated in Fig. 1. In the osimertinib group, 21 (44.7%) patients were aged ≥ 65 years, while in the afatinib group, 32 (39.5%) patients were aged ≥ 65 years. In the osimertinib group, 15 (31.9%) patients were male, while in the afatinib group, 40 (49.4%) patients were male. Most patients were nonsmokers in both groups, [33 (70.2%) in the osimertinib group, 52 (64.2%) in the afatinib group]. In the osimertinib group, 43 (91.5%) had an ECOG PS of 0–1, while in the afatinib group, 73 (90.1%) patients had an ECOG PS of 0–1. The majority of patients were stage IV in both the osimertinib and afatinib groups (83% vs 81.5%). Twenty-one (44.7%) patients with osimertinib as first-line treatment had brain metastasis at baseline, with 28 (34.6%) patients in the afatinib group experiencing the same. Regarding baseline EGFR mutation status, 26 (55.3%) patients harbored the exon 19 deletion in the osimertinib group, while similarly 35 (43.2%) patients did as well in the afatinib group. Based on the Fisher’s exact test, no significant differences were found between the osimertinib and afatinib groups regarding age, sex, smoking status, ECOG PS, clinical stage, brain metastasis at baseline, or baseline EGFR mutation status. The detailed information surrounding patient characteristics is shown in Table 1.
3.2 Clinical Efficacy of Patients with Osimertinib or Afatinib as First-Line Treatment
The date of the end of the follow-up was 22 December, 2021. The median follow-up time was 20.1 months in the osimertinib group and 22.7 months in the afatinib group. In the osimertinib group, the overall objective response rate (ORR) and disease control rate (DCR) was 66.0% and 85.1%, respectively. Amongst patients undergoing afatinib treatment, the ORR and DCR was 60.5% and 92.6%, respectively. The median PFS was 18.8 months (95% confidence interval [CI] 12.3–24.0) in the osimertinib group (n = 47) and 13.1 months (95% CI 7.7–18.5) in the afatinib group (n = 81) [log rank, p = 0.208; hazard ratio (HR) 0.45 (95% CI 0.21–0.96)] (Fig. 2A). The median OS was not reached in the osimertinib group but was 41.7 months (95% CI 29.7–53.7) in the afatinib group [log rank, p = 0.552; HR 0.79 (95% CI 0.36–1.72)] (Fig. 2B). Regarding the subtype of EGFR mutation at baseline, in patients with the exon 19 deletion, the median PFS was 31.0 months (95% CI 15.1–46.9) in the osimertinib group (n = 26) and 17.2 months (95% CI 9.2–25.2) in the afatinib group (n = 35) [log rank, p = 0.284; HR 0.68 (95% CI 0.33–1.39)] (Fig. 3A). Amongst those with the exon 21 L858R point mutation, the median PFS was 17.4 months (95% CI 6.4–28.4) in the osimertinib group (n = 21) and 10.2 months (95% CI 8.5–11.9) in the afatinib group (n = 46) [log rank, p = 0.593; HR 0.85 (95% CI 0.48–1.53)] (Fig. 3B).
3.3 Clinical Efficacy of Osimertinib and Afatinib in Patients Without Brain Metastasis at Baseline
A total of 79 patients did not have brain metastasis at baseline. The median PFS was 17.9 months (95% CI 15.1–20.7) in the osimertinib group and 17.2 months (95% CI 13.9–20.5) [n = 26] in the afatinib group (n = 53) [log rank, p = 0.962; HR 1.02 (95% CI 0.56–1.85)] (Fig. 4A). During the follow-up period, four patients (15.4%) in the osimertinib group and 12 (22.6%) in the afatinib group experienced a new development of brain metastasis. Amongst patients with the exon 19 deletion, the median PFS was not reached in the osimertinib group (n = 16) but was 21.4 months (95% CI 16.4–26.4) in the afatinib group (n = 20) [log rank, p = 0.811; HR 1.13 (95% CI 0.42–3.00)] (Fig. 4B). In patients harboring the exon 21 L858R point mutation, the median PFS was 17.9 months (95% CI 16.9–18.9) in the osimertinib group (n = 10) and 16.7 months (95% CI 8.1–25.1) in the afatinib group (n = 33) [log rank, p = 0.878; HR 1.06 (95% CI 0.49–2.31)] (Fig. 4C).
3.4 Clinical Efficacy of Osimertinib and Afatinib in Patients with Brain Metastasis at Baseline
A total of 49 patients had brain metastasis at baseline. The median PFS was 22.1 months (95% CI 2.1–42.1) in the osimertinib group (n = 21) and 10.9 months (95% CI 9.7–12.1) in the afatinib group (n = 28) [log rank, p = 0.045] (Fig. 5A). The multivariate analysis showed that osimertinib provided statistically significantly longer PFS than afatinib in patients with brain metastasis [HR 0.45 (95% CI 0.21–0.96), p = 0.038]. Concerning different subtypes of EGFR mutation, in patients having the exon 19 deletion, the median PFS was 31.0 months (95% CI 14.7–47.3) in the osimertinib group (n = 10) and 11.8 months (95% CI 9.7–13.9) in the afatinib group (n = 15) [log rank, p = 0.088; HR 0.39 (95% CI 0.13–1.20)] (Fig. 5B). Amongst patients harboring the exon 21 L858R point mutation, the median PFS was 7.1 months (95% CI 2.8–11.4) in the osimertinib group (n = 11) and 8.4 months (95% CI 5.2–11.6) in the afatinib group (n = 13) [log rank, p = 0.320; HR 0.63 (95% CI 0.25–1.60)] (Fig. 5C).
3.5 Univariate and Multivariate Analyses for PFS and OS
The univariate analysis revealed that patients with stage IVB disease (n = 65) had a worse PFS than patients with recurrence disease (n = 23), with an HR of 2.50 (95% CI 1.33–4.72; p = 0.005). Patients harboring the exon 19 deletion (n = 61) experienced a statistically lower risk of progressive disease than patients with the exon 21 L858R point mutation (n = 67), with an HR of 0.49 (95% CI 0.32–0.75; p = 0.001). The multivariate analysis confirmed both of the above results. Additionally, there was no strong evidence to present that patients receiving osimertinib as first-line treatment had better PFS than patients taking afatinib with an HR of 0.75 (95% CI 0.48–1.18; p = 0.211) (Table 2).
Regarding OS, when compared with patients with recurrent disease, patients with stage IVB disease had a higher risk of death at a HR of 12.06 (95% CI 1.58–91.97; p = 0.016), with the multivariate analysis proving this result [HR 14.14 (95% CI 1.77–113.16); p = 0.013]. No statistical difference was found in any other factors, including age, sex, smoking status, ECOG PS, status of brain metastasis at baseline, subtype of EGFR mutation, or type of EGFR-TKI used (Table 3).
3.6 Subsequent Treatment After Progressive Disease to Afatinib and Osimertinib
Up until the end date of the follow-up period, 62 patients (76.5%) experienced progressive disease during afatinib treatment. Amongst them, 44 patients (44/62, 70.1%) underwent re-biopsy, while 16 patients (16/44, 36.4%) harbored the Threonine 790 Methionine (T790M) mutation. Thirty-six patients (36/66, 58.1%) with or without T790M had received osimertinib after progressive disease occurred during afatinib treatment. Four patients (4/62, 6.5%) did not receive systemic therapy after afatinib treatment. In the osimertinib group, 28 patients (59.6%) experienced progressive disease during the follow-up period. Five patients (5/28, 17.9%) did not receive any systemic therapy after osimertinib treatment. The detailed information of sequential treatment is demonstrated in Table 4.
4 Discussion
The present research is the first study to investigate the difference in clinical efficacies between osimertinib and afatinib in a Taiwanese population. Our data have demonstrated that osimertinib as a first-line treatment established a trend showing a longer median PFS than afatinib in patients with advanced and recurrent NSCLC harboring the EGFR mutation in real-world practice. However, these results did not reach statistical significance. In a subgroup analysis, there was no difference regarding median PFS in patients without brain metastasis between the osimertinib and afatinib groups. Patients with brain metastasis receiving osimertinib as the first-line treatment experienced statistically better median PFS than those taking afatinib.
Afatinib, a second-generation EGFR-TKI, can interrupt the signal of the pan-Erb B family of receptors irreversibly [23]. The 50% inhibitory concentration of afatinib is lower than the 50% inhibitory concentration of first-generation EGFR-TKIs, including gefitinib and erlotinib, against EGFR-mutant cell lines in an in vitro study [24]. The clinical trial LUX-Lung 7 proved that patients with treatment-naive EGFR-mutant NSCLC undergoing afatinib treatment experienced longer median PFS than patients being treated with gefitinib [11.0 vs 10.9 months, HR 0.74 (95% CI 0.57–0.95), p = 0.0178] [7]. A prospective, phase IIIb, single-arm study of afatinib demonstrated that its clinical efficacies were consistent with the results from clinical trials with a median PFS of 11.4 months in a population studied in China [25]. Huang et al. confirmed the phenomenon that afatinib reduced the risk of progression when compared with first-generation EGFR-TKIs [HR 0.73 (95% CI 0.57–0.94); p =0.017] in real-world practice [11]. Furthermore, real-world data from Poland and Canada also presented that afatinib provided a more favorable OS than first-generation EGFR TKIs [26, 27].
Osimertinib, a third-generation, irreversible, selective EGFR-TKI was developed to be active against the T790M mutation, a resistance mechanism of first-generation and second-generation EGFR-TKIs. Preclinical data have revealed that osimertinib could block signaling pathways and cellular growth in both cell lines through sensitizing the EGFR mutation and druggable EGFR mutation combined with T790M in vitro [24]. AURA 3, a phase III trial, demonstrated that osimertinib provided significantly better median PFS than standard platinum-based chemotherapy in patients with advanced NSCLC harboring the T790M mutation after having acquired resistance to first-generation and second-generation EGFR-TKI treatment [10.1 vs 4.4 months, HR 0.30 (95% CI 0.23–0.41); p < 0.001] [28]. Our previous real-world data confirmed the clinical benefits of osimertinib in patients with T790M, where the median PFS and OS was 10.1 and 30.2 months, respectively [10]. In the FLAURA study, patients with treatment-naïve EGFR+ NSCLC undergoing osimertinib treatment experienced longer median PFS than patients being treated with gefitinib and erlotinib [18.9 vs 10.2 months, HR 0.46 (95% CI 0.37–0.57); p < 0.001] [13]. Furthermore, osimertinib reduced the risk of death when compared with the control group [38.6 vs 31.8 months, HR 0.80 (95.05% CI 0.64–1.00); p = 0.046] [14].
Although osimertinib has become the preferred standard first-line treatment in patients with advanced NSCLC with a sensitizing EGFR mutation, sequential treatment with first-generation and second-generation TKIs followed by osimertinib have also provided attractive results. In the GioTag study, the median time on treatment with afatinib and sequential osimertinib was 27.7 months, with the median OS being 37.6 months in patients harboring T790M after afatinib treatment [15]. Our previous study also demonstrated that the median PFS1 (PFS of gefitinib, erlotinib, or afatinib) plus PFS2 (PFS of osimertinib) was 27.5 months, while the median OS from first-line EGFR-TKI was 61.3 months amongst the 151 patients who had T790M and had received osimertinib as a subsequent treatment after experiencing resistance to first-generation and second-generation EGFR-TKIs [10]. Additionally, a subgroup analysis of the FLAURA study displayed that there was no OS benefit for patients taking osimertinib when comparing them to those being treated with first-generation TKIs in an Asian population [14]. In a FLAURA China study, although first-line osimertinib treatment provided a statistically significant median PFS benefit vs gefitinib and erlotinib [17.8 vs 9.8 months, HR 0.56 (95% CI 0.37–0.85)], there was no strong evidence to prove that osimertinib reduced the risk of death when compared with the control group in patients with advanced Chinese NSCLC with the EGFR mutation [33.1 vs 25.7 months, HR 0.85 (95% CI 0.56–1.29)] [29]. Therefore, whether first-line osimertinib treatment truly prolongs OS in an Asian population still remains uncertain. Furthermore, the comparison of clinical benefits between osimertinib and afatinib for patients with treatment-naïve EGFR-mutant NSCLC has become an important and concerning issue as well.
To clarify the difference in clinical efficacies between osimertinib and afatinib, the Heat on Beat study, a randomized phase II trial, was designed in Japan for comparing OS between afatinib and osimertinib groups in patients with treatment-naïve advanced or recurrent NSCLC with the EGFR mutation, with the results still pending [30]. Another randomized phase IV trial, AFAMOSI, was conducted in order to investigate the clinical benefits and safety of sequential afatinib and osimertinib treatment as compared to only osimertinib, as a first-line regimen in patients with EGFR(+)/T790M(−) non-squamous NSCLC (NCT04413201) [31].
Although clinical trials remain ongoing, few real-world data discussing this topic have been published. CJLSG1903, a retrospective multicenter study, enrolled patients from 15 hospitals in Japan who were being treated with either osimertinib or afatinib as a first-line therapy [16]. This study demonstrated that osimertinib did not significantly prolong the median time to discontinuation of TKIs [20.5 vs 18.6 months, HR 1.15 (95% CI 0.93–1.41); p = 0.204], time to treatment failure [20.5 vs 16.0 months, HR 0.92 (95% CI 0.76–1.13); p = 0.443], or PFS [20.5 vs 16.5 months, HR 1.02 (95% CI 0.81–1.28), p = 0.864], when compared with afatinib. Moreover, it presented that osimertinib had a statistically significantly higher risk of death when compared with afatinib [25.1 vs 36.2 months, HR 1.47 (95% CI 1.07–2.02); p = 0.018]. However, the median follow-up time was not equal in the afatinib and osimertinib groups (26.2 vs 9.4 months). The event rate was 73.5% in the afatinib group, but only 29.4% in the osimertinib group. Therefore, we should interpret these results cautiously. In another real-world retrospective study, Mitsuya et al. reported that there were no significant differences in both median PFS [not reached vs 23 months, HR 0.932 (95% CI 0.379–2.287); p = 0.877] and OS [33 vs 36 months, HR 2.917 (95% CI 0.780–10.905); p = 0.112] to be found between the osimertinib and afatinib groups in patients with advanced or recurrent EGFR-mutated NSCLC [17]. However, this study only enrolled 49 patients for analysis, and the median follow-up time was not the same. Our present study has demonstrated that patients receiving osimertinib (n = 47) as a first-line treatment did not experience statistically prolonged PFS [18.8 vs 13.1 months, HR 0.75 (95% CI 0.48–1.18); p = 0.211] or OS [not reached vs. 41.7 months, HR 0.79 (95% CI 0.36–1.72); p = 0.553], when compared to those treated with afatinib (n = 81). Although the HRs of both PFS and OS are in favor of osimertinib as a first-line treatment, the results did not reach statistical significance. This condition may be a result of the small number of patients we enrolled for analysis. In our study, the median follow-up time was 20.1 months in the osimertinib group and 22.7 months in the afatinib group without any statistical difference being evident (p = 0.413). Additionally, the event rates of PFS and OS were relatively close in the two groups (osimertinib vs afatinib; PFS 59.6% vs 75.3%; OS 19.1% vs 27.2%). Therefore, we believe that our findings are more powerful than those seen in other studies.
Regarding the status of brain metastasis at baseline, CJLSG1903 showed that osimertinib provided a trend towards a longer median PFS (HR 0.60; p = 0.062), as well as time to discontinuation of TKI (HR 0.66; p = 0.0103), than afatinib did in patients with brain metastasis [16]. The findings from the present study are consistent with the above results. Amongst patients with brain metastasis in our research study, patients in the osimertinib group (n = 21) experienced significantly superior median PFS than patients in the afatinib group (n = 28) [22.1 vs. 10.9 months, HR 0.45 (95% CI 0.21–0.96); p = 0.038]. Alternatively, there were no differences in median PFS between the osimertinib (n = 26) and afatinib (n = 53) groups in patients without brain metastasis [17.9 vs 17.2 months; HR 1.02 (95% CI 0.56–1.85); p = 0.962]. In preclinical data, osimertinib also demonstrated significantly better brain penetration than first-generation and second-generation EGFR-TKIs through in vitro and in vivo models [32, 33]. According to the preclinical data and clinical results, osimertinib as first-line therapy may be a favorable choice in patients with EGFR-mutant NSCLC with brain metastasis. Otherwise, a subgroup analysis from our study involving an exon 19 deletion with brain metastasis (n = 25), an exon 19 deletion without brain metastasis (n = 36), an L858R mutation with brain metastasis (n = 24), and an L858R mutation without brain metastasis (n = 43) did not reveal a difference in median PFS between the two groups. However, the population in each subgroup was too small, making it difficult to draw conclusions from these data.
Finally, in the present study, we demonstrated through univariate and multivariate analyses that patients with stage IVB disease experienced significantly worse PFS and OS than patients with recurrent disease. However, patients without brain metastasis did not have significantly better PFS and OS than patients with brain metastasis. Actually, there was still a trend to imply the phenomenon with an HR of 0.72 (95% CI 0.47–1.10) and an HR of 0.61 (95% CI 0.30–1.26), respectively. Additionally, it seems that patients with ECOG PS 0–1 did not experience longer PFS and OS than patients with ECOG PS 2–3. These results may have been caused by the extremely unequal patient numbers (116 patients in the PS 0–1 group vs 12 in the PS 2–3 group).
Although our research was the first study in Taiwan to compare the difference in clinical efficacy between osimertinib and afatinib in patients with advanced and recurrent EGFR-mutant NSCLC, there were still some limitations that should be acknowledged. First, the present research was a single-center retrospective study, thus more bias would therefore be present when compared with other studies that are prospectively designed. Second, only Taiwanese individuals were eligible for analysis. Therefore, our findings may not be generalizable to other ethnic populations. Third, because of the fact that the enrolled population was relatively small, we should interpret the data from the subgroup analysis cautiously. Fourth, the follow-up time was not long enough, thus it is difficult to discuss OS-related issues. Fifth, 25% of patients in the osimertinib group and 8.1% of patients in the afatinib group did not receive sequential treatment after progressive disease to first-line EGFR-TKI therapy. The condition might influence the results of OS. Finally, because previous trials had displayed the adverse events of each drug clearly, we did not emphasize this topic [7, 13].
5 Conclusions
Our findings shed light on the differences in clinical efficacies between patients receiving osimertinib and afatinib as first-line treatment in treatment-naïve advanced and recurrent NSCLC with the EGFR mutation. Our research has demonstrated that there was no significant difference in median PFS and OS between the osimertinib and afatinib groups. However, osimertinib provided statistically longer median PFS than afatinib in patients with brain metastasis. Nevertheless, we believe further results from additional clinical trials for the purpose of confirming our findings are still required.
Change history
05 November 2022
A Correction to this paper has been published: https://doi.org/10.1007/s11523-022-00928-4
References
Kris MG, Johnson BE, Berry LD, Kwiatkowski DJ, Iafrate AJ, Wistuba II, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311:1998–2006.
Shi Y, Au JS, Thongprasert S, Srinivasan S, Tsai CM, Khoa MT, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol. 2014;9:154–62.
Hsu KH, Ho CC, Hsia TC, Tseng JS, Su KY, Wu MF, et al. Identification of five driver gene mutations in patients with treatment-naïve lung adenocarcinoma in Taiwan. PLoS One. 2015;10:e0120852.
Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57.
Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–46.
Sequist LV, Yang JC, Yamamoto N, O’Byrne K, Hirsh V, Mok T, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3327–34.
Park K, Tan EH, O’Byrne K, Zhang L, Boyer M, Mok T, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol. 2016;17:577–89.
Wu YL, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18:1454–66.
Mok TS, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, et al. Updated overall survival in a randomized study comparing dacomitinib with gefitinib as first-line treatment in patients with advanced non-small-cell lung cancer and EGFR-activating mutations. Drugs. 2021;81:257–66.
Huang YH, Tseng JS, Hsu KH, Chen KC, Su KY, Yu SL, et al. The impact of different first-line EGFR-TKIs on the clinical outcome of sequential osimertinib treatment in advanced NSCLC with secondary T790M. Sci Rep. 2021;11:12084.
Huang AC, Huang CH, Ju JS, Chiu TH, Tung PH, Wang CC, et al. First- or second-generation epidermal growth factor receptor tyrosine kinase inhibitors in a large, real-world cohort of patients with non-small cell lung cancer. Ther Adv Med Oncol. 2021;13:17588359211035710.
Su VY, Yang KY, Huang TY, Hsu CC, Chen YM, Yen JC, et al. The efficacy of first-line tyrosine kinase inhibitors combined with co-medications in Asian patients with EGFR mutation non-small cell lung cancer. Sci Rep. 2020;10:14965.
Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378:113–25.
Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382:41–50.
Hochmair MJ, Morabito A, Hao D, Yang CT, Soo RA, Yang CH, et al. Sequential afatinib and osimertinib in patients with EGFR mutation-positive non-small-cell lung cancer: final analysis of the GioTag study. Future Oncol. 2020;16:2799–808.
Ito K, Morise M, Wakuda K, Hataji O, Shimokawaji T, Takahashi K, et al. A multicenter cohort study of osimertinib compared with afatinib as first-line treatment for EGFR-mutated non-small-cell lung cancer from practical dataset: CJLSG1903. ESMO Open. 2021;6:100115.
Mitsuya S, Tsuruoka K, Kanaoka K, Funamoto T, Tsuji H, Matsunaga N, et al. Comparison between second- and third-generation epidermal growth factor receptor tyrosine kinase inhibitors as first-line treatment in patients with non-small-cell lung cancer: a retrospective analysis. Anticancer Res. 2021;41:513–45.
Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, et al. AJCC cancer staging manual. 8th ed. New York: Springer; 2017.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Tsai TH, Su KY, Wu SG, Chang YL, Luo SC, Jan IS, et al. RNA is favourable for analysing EGFR mutations in malignant pleural effusion of lung cancer. Eur Respir J. 2012;39:677–84.
Su KY, Chen HY, Li KC, Kuo ML, Yang JC, Chan WK, et al. Pretreatment epidermal growth factor receptor (EGFR) T790M mutation predicts shorter EGFR tyrosine kinase inhibitor response duration in patients with non-small-cell lung cancer. J Clin Oncol. 2012;30:433–40.
Su KY, Tseng JS, Liao KM, Yang TY, Chen KC, Hsu KH, et al. Mutational monitoring of EGFR T790M in cfDNA for clinical outcome prediction in EGFR-mutant lung adenocarcinoma. PLoS One. 2018;13:e0207001.
Solca F, Dahl G, Zoephel A, Bader G, Sanderson M, Klein C, et al. Target binding properties and cellular activity of afatinib (BIBW 2992), an irreversible ErbB family blocker. J Pharmacol Exp Ther. 2012;343:342–50.
Cross DA, Ashton SE, Ghiorghiu S, Eberlein C, Nebhan CA, Spitzler PJ, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014;4:1046–61.
Tu HY, Feng J, Shi M, Zhao J, Wang Y, Chang J, et al. A phase IIIb open-label, single-arm study of afatinib in EGFR TKI-naïve patients with EGFRm+ NSCLC: final analysis, with a focus on patients enrolled at sites in China. Target Oncol. 2022;17:1–13.
Pluzanski A, Krzakowski M, Kowalski D, Dziadziuszko R. Real-world clinical outcomes of first-generation and second-generation epidermal growth factor receptor tyrosine kinase inhibitors in a large cohort of European non-small-cell lung cancer patients. ESMO Open. 2020;5:e001011.
Lau SC, Chooback N, Ho C, Melosky B. Outcome differences between first- and second-generation EGFR inhibitors in advanced EGFR mutated NSCLC in a large population-based cohort. Clin Lung Cancer. 2019;20:e576–83.
Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376:629–40.
Cheng Y, He Y, Li W, Zhang HL, Zhou Q, Wang B, et al. Osimertinib versus comparator EGFR TKI as first-line treatment for EGFR-mutated advanced NSCLC: FLAURA China, a randomized study. Target Oncol. 2021;16:165–76.
Morikawa K, Tanaka H, Itani H, Takata S, Watanabe S, Kishi K, et al. Hypothesis generative head-to-head study comparing efficacy of afatinib and osimertinib based on immunological biomarkers in Japanese NSCLC patients with EGFR mutations (Heat on Beat study). Ther Adv Med Oncol. 2020;12:1758835920967254.
AFAMOSI: efficacy and safety of afatinib Followed by osimertinib compared to osimertinib in patients with EGFRmutated/T790M mutation negative nonsquamous NSCLC. Available from: https://ClinicalTrials.gov/show/NCT04413201. Accessed 1 Feb 2022.
Ballard P, Yates JW, Yang Z, Kim DW, Yang JC, Cantarini M, et al. Preclinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res. 2016;22:5130–40.
Colclough N, Chen K, Johnström P, Strittmatter N, Yan Y, Wrigley GL, et al. Preclinical comparison of the blood-brain barrier permeability of osimertinib with other EGFR TKIs. Clin Cancer Res. 2021;27:189–201.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
No external funding was used in the preparation of this article.
Conflict of interest
Yen-Hsiang Huang, Kuo-Hsuan Hsu, Jeng-Sen Tseng, Tsung-Ying Yang, Kun-Chieh Chen, Kang-Yi Su, Sung-Liang Yu, Jeremy J.W. Chen, and Gee-Chen Chang all declare that they have no conflicts of interest that might be relevant to the contents of this article.
Ethics approval
The study was approved by the Institutional Review Board of Taichung Veterans General Hospital, Taiwan (No. CF12019).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Authors’ contributions
Yen-Hsiang Huang, Tsung-Ying Yang, Jeremy J.W. Chen, and Gee-Chen Chang contributed to the study conception and design. Material preparation and data collection were performed by Yen-Hsiang Huang, Kuo-Hsuan Hsu, Jeng-Sen Tseng, Tsung-Ying Yang, Kun-Chieh Chen, and Gee-Chen Chang. The analysis of the data was done by Yen-Hsiang Huang, Tsung-Ying Yang, Kang-Yi Su, Sung-Liang Yu, Jeremy J.W. Chen, and Gee-Chen Chang. The writing of the original draft was performed by Yen-Hsiang Huang, Kuo-Hsuan Hsu, Jeng-Sen Tseng, and Kun-Chieh Chen. Tsung-Ying Yang, Kang-Yi Su, Sung-Liang Yu, Jeremy J.W. Chen, and Gee-Chen Chang revised the manuscript critically for important intellectual content.
Additional information
The original article has been updated: Due to retrospective open choice order.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Huang, YH., Hsu, KH., Tseng, JS. et al. The Difference in Clinical Outcomes Between Osimertinib and Afatinib for First-Line Treatment in Patients with Advanced and Recurrent EGFR-Mutant Non-Small Cell Lung Cancer in Taiwan. Targ Oncol 17, 295–306 (2022). https://doi.org/10.1007/s11523-022-00878-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-022-00878-x