Abstract
Background
Gemcitabine plus sirolimus enhances apoptosis in vitro and increases anti-tumor efficacy in vivo in soft-tissue sarcoma (STS) models.
Objective
The objective of this study was to evaluate the activity and toxicity of the combination of gemcitabine plus sirolimus in patients with STS after failure of standard chemotherapy.
Patients and Methods
Advanced STS patients, previously treated with doxorubicin and/or ifosfamide, were included in this single-arm phase II study. Patients received gemcitabine 800 mg/m2 intravenously (iv) at 10 mg/m2/min on days 1 and 8 every 3 weeks plus sirolimus 5 mg daily orally (po). After enrolment of the first 12 patients, the study protocol was amended due to toxicity and the starting dose of sirolimus was reduced to 3 mg daily po. Archival tumor samples were analyzed for extracellular signal-regulated kinase 1 and 2 (ERK1/2) expression and correlated with outcome. The primary endpoint was progression-free rate (PFR) at 3 months.
Results
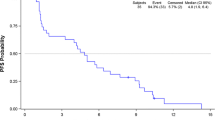
From May 2012 to May 2013, 28 patients were enrolled at eight centers. PFR at 3 and 6 months was 44% and 20%, respectively, with 12 patients being free of progression at 3 months. Median progression-free survival (PFS) was 1.85 months (95% confidence interval [CI] 0.73–2.97) and median overall survival (OS) was 9.2 months (95% CI 5.8–12.5). No responses were observed. The most common grade 3–4 hematologic toxicities were neutropenia (48%) and leukopenia (41%) and the most frequent grade 3 non-hematologic toxicities were infection (18.5%), transaminitis (15%), fatigue (11%), and pneumonitis (11%). ERK1/2 expression was significantly correlated with PFS (p = 0.026).
Conclusions
The combination of gemcitabine and sirolimus is an active treatment in STS. Further investigation is warranted. ClinicalTrials.gov identifier: NCT01684449.


Similar content being viewed by others

References
Van Glabbeke M, van Oosterom AT, Oosterhuis JW, Mouridsen H, Crowther D, Somers R, et al. Prognostic factors for the outcome of chemotherapy in advanced soft tissue sarcoma: an analysis of 2,185 patients treated with anthracycline-containing first-line regimens—a European Organization for Research and Treatment of cancer soft tissue and bone sarcoma group study. J Clin Oncol. 1999;17(1):150–7.
van Oosterom AT, Mouridsen HT, Nielsen OS, Dombernowsky P, Krzemieniecki K, Judson I, et al. Results of randomised studies of the EORTC soft tissue and bone sarcoma group (STBSG) with two different ifosfamide regimens in first- and second-line chemotherapy in advanced soft tissue sarcoma patients. Eur J Cancer. 2002;38(18):2397–406.
Leahy M, Garcia Del Muro X, Reichardt P, Judson I, Staddon A, Verweij J, et al. Chemotherapy treatment patterns and clinical outcomes in patients with metastatic soft tissue sarcoma. The SArcoma treatment and burden of illness in North America and Europe (SABINE) study. Ann Oncol. 2012;23(10):2763–70.
Tap WD, Jones RL, Van Tine BA, Chmielowski B, Elias AD, Adkins D, et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Lancet. 2016;388(10043):488–97.
Späth-Schwalbe E, Genvresse I, Koschuth A, Dietzmann A, Grunewald R, Possinger K. Phase II trial of gemcitabine in patients with pretreated advanced soft tissue sarcomas. Anti-Cancer Drugs. 2000;11(5):325–9.
Patel SR, Gandhi V, Jenkins J, Papadopolous N, Burgess MA, Plager C, et al. Phase II clinical investigation of gemcitabine in advanced soft tissue sarcomas and window evaluation of dose rate on gemcitabine triphosphate accumulation. J Clin Oncol. 2001;19(15):3483–9.
Svancárová L, Blay JY, Judson IR, van Hoesel QG, van Oosterom AT, le Cesne A, et al. Gemcitabine in advanced adult soft-tissue sarcomas. A phase II study of the EORTC soft tissue and bone sarcoma group. Eur J Cancer. 2002;38(4):556–9.
Hartmann JT, Oechsle K, Huober J, Jakob A, Azemar M, Horger M, et al. An open label, non-comparative phase II study of gemcitabine as salvage treatment for patients with pretreated adult type soft tissue sarcoma. Investig New Drugs. 2006;24(3):249–53.
Ferraresi V, Ciccarese M, Cercato MC, Nuzzo C, Zeuli M, Di Filippo F, et al. Gemcitabine at fixed dose-rate in patients with advanced soft-tissue sarcomas: a mono-institutional phase II study. Cancer Chemother Pharmacol. 2008;63(1):149–55.
Pautier P, Floquet A, Penel N, Piperno-Neumann S, Isambert N, Rey A, et al. Randomized multicenter and stratified phase II study of gemcitabine alone versus gemcitabine and docetaxel in patients with metastatic or relapsed leiomyosarcomas: a federation Nationale des Centres de Lutte Contre le cancer (FNCLCC) French sarcoma group study (TAXOGEM study). Oncologist. 2012;17(9):1213–20.
Ducoulombier A, Cousin S, Kotecki N, Penel N. Gemcitabine-based chemotherapy in sarcomas: a systematic review of published trials. Crit Rev Oncol Hematol. 2016;98:73–80.
García-Del-Muro X, López-Pousa A, Maurel J, Martín J, Martínez-Trufero J, Casado A, et al. Randomized phase II study comparing gemcitabine plus dacarbazine versus dacarbazine alone in patients with previously treated soft tissue sarcoma: a Spanish Group for Research on sarcomas study. J Clin Oncol. 2011;29(18):2528–33.
Maki RG, Wathen JK, Patel SR, Priebat DA, Okuno SH, Samuels B, et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected]. J Clin Oncol. 2007;25(19):2755–63.
Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441(7092):424–30.
Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2(7):489–501.
Demetri GD, Chawla SP, Ray-Coquard I, Le Cesne A, Staddon AP, Milhem MM, et al. Results of an international randomized phase III trial of the mammalian target of rapamycin inhibitor ridaforolimus versus placebo to control metastatic sarcomas in patients after benefit from prior chemotherapy. J Clin Oncol. 2013;31(19):2485–92.
Martin-Liberal J, Gil-Martín M, Sáinz-Jaspeado M, Gonzalo N, Rigo R, Colom H, et al. Phase I study and preclinical efficacy evaluation of the mTOR inhibitor sirolimus plus gemcitabine in patients with advanced solid tumours. Br J Cancer. 2014;111(5):858–65.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47.
Van Glabbeke M, Verweij J, Judson I, Nielsen OS. Group ESTaBS. Progression-free rate as the principal end-point for phase II trials in soft-tissue sarcomas. Eur J Cancer. 2002;38(4):543–9.
West KA, Castillo SS, Dennis PA. Activation of the PI3K/Akt pathway and chemotherapeutic resistance. Drug Resist Updat. 2002;5(6):234–48.
LoPiccolo J, Blumenthal GM, Bernstein WB, Dennis PA. Targeting the PI3K/Akt/mTOR pathway: effective combinations and clinical considerations. Drug Resist Updat. 2008;11(1–2):32–50.
Martin-Broto J, Redondo A, Valverde C, et al. Gemcitabine plus sirolimus for relapsed and progressing osteosarcoma patients after standard chemotherapy: a multicenter, single-arm phase II trial of Spanish Group for Research on Sarcoma (GEIS). Ann Oncol. Epub 2017 Sep 25. https://doi.org/10.1093/annonc/mdx536.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by Grant TRA-163 from the Spanish Ministry of Health.
Conflict of Interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Martin-Liberal, J., López-Pousa, A., Martínez-Trufero, J. et al. Phase II Study of Gemcitabine Plus Sirolimus in Previously Treated Patients with Advanced Soft-Tissue Sarcoma: a Spanish Group for Research on Sarcomas (GEIS) Study. Targ Oncol 13, 81–87 (2018). https://doi.org/10.1007/s11523-017-0539-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-017-0539-9