Abstract
The main rationale for using biosimilar drugs is for cost saving. The market development for biosimilar drugs will therefore depend on the degree to which cost saving measures are required by nations, medical insurers and individuals and the absolute savings that could be gained by switching from original drugs. This paper is designed to discover the degree to which financial constraints will drive future health spending and to discover if legal or safety issues could impact on any trend. A structured literature search was performed for papers and documents to 27 August 2011. Where multiple sources of data were available on a topic, data from papers and reports by multinational or national bodies were used in preference to data from regions or individual hospitals. Almost all health systems face current significant cost pressures. The twin driver of increasing cancer prevalence as populations age and cancer medicine costs rising faster than inflation places oncology as the most significant single cost problem. For some countries, this is predicted to make medicine unaffordable within a decade. Most developed countries have planned to embrace biosimilar use as a cost-control measure. Biosimilar introduction into the EU has already forced prices down, both the price of biosimilar drugs and competitive price reductions in originator drugs. Compound annual growth rates of use have been predicted at 65.8% per year. Most developed countries have planned to embrace biosimilar use as a major cost-control measure. Only legal blocks and safety concerns are likely to act against this trend. For centralised healthcare systems, and those with a strong tradition of generic medicine use, biosimilar use will clearly rise with predictions of more than 80% of prescriptions of some biologic drugs within 1 year of market entry in the USA. Delaying the implementation of such programmes however risks a real crisis in healthcare delivery for many countries and hospitals that few can now afford.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
The main rationale for using biosimilar drugs, rather than the original version, is for cost saving. The market development for biosimilar drugs will therefore depend on the degree to which cost-saving measures are required by nations, medical insurers and individuals, and the absolute savings that could be gained by switching from original drugs. This paper is designed to discover the degree to which financial constraints will drive future health spending, and to discover if legal or safety issues could impact on any trend in biosimilar use.
The world population growth and population ageing predict a progressive increase in cancer prevalence [1]. The World Health Organization (WHO) predicted cancer would be the world’s leading cause of death by 2010 [2]. While demographic changes drove up the prevalence of cancer, cancer research delivered more potential treatments that patients could use. As a result, the American cancer drugs budget rose four-fold in the decade 1998 to 2008. Over that time, cancer medicines became the best-selling class of drug in the United States, having surpassed lipid regulators [3]. Many of the new generation of biologic cancer therapies cost $100,000 per treatment course on an annual basis [4]. This increase in costs may be unsustainable for even wealthy countries. In the United States, Medicare costs, which fund care to the over 65-year age group, are projected to grow at unprecedented levels over the next few decades, from 11% in 2010 to 24% of all tax revenue by 2030 [5]. In the context of rising demand for medical treatment and increasing healthcare costs, the development of “biosimilar” or “biologic follow-on medication” may provide a route for cost savings.
Methods
A structured literature search was performed for papers and documents up to 27 August 2011. The Medical Subject Headings (MeSH) portal of the USA National Library of Congress’ PubMed database was the primary search portal [6]. As of 27 August 2011, the term “biosimilar” was not included [7]. Using the MeSH terms “(”Cost–Benefit Analysis”[Majr]) AND (“Neoplasms”[Mesh])” revealed 244 publications of which 42 were reviews. Related papers were identified using the “PubMed” related papers system and by manual search of references from within these papers. Web-based documents were searched using the Google Advanced search engine using the terms “Cost–Benefit Analysis AND Neoplasms OR cancer.” When a relevant web page was found, it was searched again for related content with the Google Related sites program [8].
Data on likely market share by biosimilars was searched using the Google Advanced search engine using the exact phrase “market share for biosimilars.” This revealed 31 web pages up to 13 March 2011. When a relevant web page was found, it was searched again for related content with the Google “related sites” program [8]. Where relevant scientific papers were identified or referenced in web pages or documents, these were located in “PubMed,” and again, related papers were identified using the”PubMed” related-papers system and by manual search of references from within these papers.
Where multiple sources of data were available on a topic, data from papers and reports by multinational or national bodies were used in preference to data from regions or individual hospitals.
Results
The literature search strategy revealed papers and documents in several grouped themes. The first major theme was the problem that rising demand for cancer treatment and cancer treatment costs give to health systems. The second theme was the current solutions in place to manage these cost demands. The last theme was the role that biologic and biosimilar drugs would play in future cancer medicine.
While there was good agreement between studies on the first two themes, predictions on the future use of biosimilars differed between papers from within and outside the US.
The magnitude of the problem—population demographics
The incidence of cancer in people above the age 65 is nearly 10 times that of people below 65. As the populations age, health systems and insurers have found themselves diverting steadily more resources to pay for cancer treatments [9]. Cancer is now the leading cause of death in many countries. The WHO estimated it had become the world’s leading cause of death in 2010 [10]. The prediction of the world cancer burden, projected from population growth and ageing, suggested 15 million new cases and 10 million new deaths were expected in 2020 [1]. This was based on a predicted annual rise of 1% in cases and deaths, with even greater increases expected in China, Russia, and India. The WHO estimated that new cancer cases would probably increase to 27 million annually by 2030, with deaths reaching 17 million each year. For a small, developed European country such as Ireland, the total number of new cancers is predicted to almost double in two decades (1998–2002 and 2020) [11]. In comparison, for wealthy but developing countries with a young age profile, such as Saudi Arabia, in two decades the cancer burden is expected to grow 8 to 10 fold [12].
The magnitude of the problem—financial impact
Cancer medicine is, in many countries, the leading driver of increased healthcare costs. For example, direct medical spending for cancer in the USA was $104 billion in 2010. It has risen 222% in 20 years, which is faster than any other branch of medicine in developed countries over the same era [13]. This threat of growing healthcare costs is not new, but it impacts increasingly at both a personal and family level and on national economic policies. Back in 1980, the US News and World Report magazine wrote that healthcare costs would rise at an exponential rate, increasing 50-fold in the 40 years between 1950 and 1990, from $12.75 billion to $757.9 billion [14]. As a share of the USA GDP, the national health expenditure has risen from 5.2% in 1960 to 16.2% in 2007 [15]. Over that period, health costs rose from fifth to first in the family budget [16]. The impact of this medical advance but financial threat is seen in increased insurance premiums. In the USA, medical insurance costs have risen faster than earnings and general inflation; shown graphically in Fig. 1 [17]. This widening gap in health spends and earnings will eventually become unsustainable [18] (Fig. 1).
The growth in insurance premiums and employee contributions to health insurance compared with employee earnings and general inflation in the USA over time from 1999 to 2010. Data from the Kaiser/HRET Survey of Employer-Sponsored Health Benefits, 1999-2010. Bureau of Labor Statistics, Consumer Price Index, U.S. City Average of Annual Inflation (April to April), 1999-2010; Bureau of Labor Statistics, Seasonally Adjusted Data from the Current Employment Statistics Survey, 1999-2010 (April to April)
The consequence of the rising cost of cancer treatment is threats to the financial solvency of patients [19, 20]. Medical insurance premiums in the USA have more than doubled since 1999 (131% increase) [21]. A study by the American Cancer Society found that one in five families used up all of their savings paying for cancer treatment [22]. Another showed that 62% of all USA bankruptcies came from medical expenses [23]. To continue providing medical care to retired citizens, US Medicare costs are projected to grow at unprecedented levels over the next few decades [24]. They are predicted to consume 24% of all tax revenue by 2030, more than doubling from 11% in 2010. Using data from the US Centers for Medicare and Medicaid, the Office of the Actuary and the US National Health Statistics Group, Sean Keehan points out that the gap between the large rises in the US National Health expenditure and smaller rises in national wealth (GDP) will eventually make healthcare unaffordable [25]. The current 2% gap in rises means that real living standards will fall in the US, as spending on things other than health is squeezed, prompting a crisis in health funding within a decade [26].
Cost problems in cancer care are universal. In the Republic of Korea, life expectancy has risen from 64.8 to 78.5 years in 30 years, to reach the OECD average. Cancer patients, whose treatment might involve multiple surgical interventions, chemotherapy and a prolonged period of hospitalization, may face huge bills as the Korean National Health Insurance scheme covers only 75% of the cost. The Republic of Korea has the highest out-of-pocket spending of any OECD country, with 36% of total health expenditure coming directly from patient payments at the point of service in 2007. Inevitably, this results in unaffordable bills for some. In 2007, an estimated 3% of all households in the country suffered catastrophic expenditure, defined by the WHO as an obligatory disbursement greater than or equal to 40% of residual household income after basic needs have been met [27].
The magnitude of the problem—cancer drug costs
Cancer is the growth area for novel medicines. Between July 2005 and December 2007, the US Food and Drugs Administration (FDA) approved 53 new indications in oncology, with 18 new molecular entity approvals [28]. The FDA has also seen a huge increase in investigational agents studied in cancer, from 925 investigational new drug applications in 2003 to 1440 in 2008. Innovative drug development is slow and expensive. From 5000 to 10,000 compounds in pre-clinical trials, only 0.1% reach clinical trial stage and of these, only 10–20% are finally approved with a typical development time of 15 years [29]. The high cost of bringing a novel biologic drug to market has been estimated at $800 million in 2006. As a result, the American cancer drugs budget rose four-fold in the decade 1998 to 2008 [3, 30]. During that period, cancer medicines surpassed lipid regulators to become the best-selling class of drug.
Ambulatory cancer care seems to be the driver for the increase in costs. The US Medicare spending on drugs administered in a doctor’s office, the vast majority of which are cancer treatments, rose from$3 billion in 1997 to $11 billion in 2004, a 267% increase while overall Medicare spending rose by only 47% over the same period [31]. The American data is confirmed in Europe. In France, the cancer drugs budget has been doubling every 4 years, rising from €474 million in 2004 to €975 million in 2008 [32].
Most novel cancer drugs are high-cost biologics. These include imatinib for Chronic Myeloid Leukaemia, trastuzumab for HER2-overexpressing breast cancer, and rituximab for B-cell lymphomas. All have demonstrated the benefits of the investment in translational research in basic cancer science. However, with these advances have come problems. The principal concern emerges with the cost of treatment [33]. Because of the innovative but expensive research and regulation, the cost of novel cancer drugs has risen by the year of approval [34]. According to a recent report in the Journal of the National Cancer Institute, 90% of cancer-fighting drugs or biologics approved by the FDA over the past 4 years cost more than $20,000 for a 12-week course of therapy, with many offering a survival benefit of only 2 months or less [35]. As an example of the effect of novel drugs on the costs of cancer care, the cost of treatment using standard chemotherapy regimens evidenced by randomized trials for metastatic colon cancer was compared over time [36]. Costs were estimated at 95% of the average wholesale drug price for May 2004. Using the Mayo clinic regimen of 5-flurouracil and leucovorin as a benchmark at $63 for drugs for each 8-week treatment the costs rose with each improvement. Second-generation regimens containing irinotecan or oxaliplatin cost $9497 to $11,899 for an 8-week treatment, while third generation regimens containing bevacizumab or cetuximab cost $21,339 to $30,790. The rise from $63 to $30,790 represented an almost five hundred-fold rise in drug cost (30,790/63 = 488.730159). While some argue that this cost represents value for the improved outcomes, others point out that the clinical benefits are not proportionate to the rise in cost of the drug [37, 38] (Table 1).
The magnitude of the problem—the balance between increased treatment costs and population demographics
While novel drug innovation over time may be associated with reduced cancer death rates, the rising treatment costs are compounded by demographics [39, 40]. In all societies, the growth and ageing population is associated with more cancer to treat. Knowing the relative balance between the twin drivers of future costs of more cancer patients and increasingly expensive novel biologic treatments will help to predict whether cost-containment program can deliver better healthcare at reduced costs.
The US National Cancer Institute has provided a website for investigators to review their cancer prevalence and cost of care projection data [41]. Based on growth and aging of the US population, medical expenditures for cancer in the year 2020 are projected to reach at least $158 billion (in 2010)—an increase of 27% over 2010, according to a National Institutes of Health analysis [42]. Researchers from the National Cancer Institute (NCI) use this to predict that if new tests for cancer diagnosis, novel treatment, and follow-up continue to be more expensive, medical expenditures for cancer could reach as high as $207 billion. Though increases in the number of beneficiaries account for some of the increase in the cost of the Medicare program, new technology is estimated to account for up to 48% of the change in spending since 1960 [43].
For a developed country, such as the US, the Office of Management and Budget predicts more costly healthcare per patient dominates as the driver for rising overall healthcare costs. In contrast, the aging of the population accounts only for a modest fraction of the projected growth in federal spending on Medicare and Medicaid. This is shown graphically in Fig. 2. This is useful information, as it gives hope that cost-control programs can contribute to affordable future health care as there would be little that could be done in the face of unchangeable demographics (Fig. 2).
The relative effect of demographics and increased unit treatment costs on the spending on Medicare, Medicaid as a% of USA over time from 2007 to 2082. The aging of the population accounts only for a modest fraction of the projected growth in federal spending on Medicare and Medicaid, while costs increasing at current rates push spending to almost 20% of USA GDP by 2082. Data from—The long-term outlook for health care spending, A Congressional Budget Office study, Nov 2007, Pub No. 3085; page 14
The magnitude of the problem—interventions that could be implemented at a national level
Oncologists have a duty of care to future as well as current patients. We are reminded by the WHO “to require the health system to obtain the greatest possible level of health from the resources devoted to it.” This is “to ask that it be as cost-effective as it can be” [44]. Concentration on cost alone is not enough. Simplistic economic assessments concentrate on limiting access to high-cost treatments by rationing. This, however, misses the point that many cheap but common high-volume treatments may be relatively ineffective, whereas some expensive novel medicines are highly effective and save money elsewhere in the health system; by increasing cures or by saving money from other healthcare budgets. For example, trastuzumab (a monoclonal antibody) costs about $70,000 for a full course of breast cancer adjuvant treatment and is associated with a 52% reduction in disease recurrence and 33% reduction in death [45, 46]. Over a lifetime, the cost for each extra quality adjusted life year (QALY) is estimated at $27,800 (range $18,000–39,000) [47]. In contrast, extending the hospital stay of myocardial infarction patients beyond 4 days costs $105,000 per QALY gained [48].
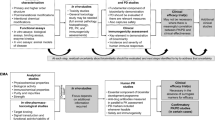
Physicians need to be reassured that economics is not primarily about saving money. It is about using scarce resources as efficiently as possible. Economists never say “cheap” or “expensive”—they say “cost-effective” or “not cost effective.” Since rationing occurs in every health system, either by personal ability to pay or by society’s willingness to pay it, physicians need to understand how economic studies can contribute to improved patient well being and health. To understand the cost-effectiveness of treatments, we need to know both costs and effects. Costs to consider are of treating, of not treating, and of alternate treatments. Effects include the benefits seen, their magnitude and duration. Balanced against them are the treatment toxicities seen, their magnitude and duration. For a costs effectiveness study, the balance requires single metric of costs and a single metric of risks/benefits to the length and quality of life. In practice, this has now become the incremental cost effectiveness ratio (ICER). This is the monetary cost required to gain an extra Quality Adjusted Life Year (QALY) from each treatment [49, 50]. The derivation of a QALY is shown graphically in Fig. 3. As an example of QALY calculations, an intervention that lengthened life for a whole year at 100% full quality of life (QOL) would have gained 1 QALY (1 × 1 = 1 QALY). Another intervention that gained 6 months extra life lived at 50% of normal full QOL would have gained only 0.25 of a QALY (0.5 × 0.5 = 0.25 QALY). Another that gained 2 extra years at 75% of full QOL would have gained 1.5 QALY (2 × 0.75 = 1.5) (Fig. 3).
A diagram to represent the QALY effect. Treatment A increases length and quality of life over control. Treatment B increases only quality of life over treatment A. Treatment B is clinically better than treatment A (it gains more QALYs than treatment B) but the ICERs of the two different interventions are required to decide which is the most cost-effective option?
While it may appear initially cruel to set a threshold ICER above which new treatments are not approved or reimbursed, policy makers will generally have aims that physicians would support. The policy aims to protect and improve the health of the population, to assure access to medical care, to achieve efficient use of healthcare resources, and thus control the rate of growth of expenditures for medical care to ensure that a sudden funding crisis does not occur, which threatens the basic medical provision. The World Health Report for 2000 makes it clear that money itself does not buy health or longevity [51]. To achieve life expectancy between 78 and 82 years, the 191 WHO member states spend between $2000 and $6000 per capita on health. In this range, there is only a weak correlation between spend and longevity. When freedom from disability was studied, the range of spend varied more than ten-fold.
It becomes increasingly important that when a treatment has a low cost effectiveness, physicians should ask is it worth doing compared to other things we could do with the same money? Assuming we worry about costs, and that some publicly funded healthcare is essential, it is reasonable to ask could we perform better? In the US, there are 185 publicly funded interventions which together cost about $21.4 billion per year, for an estimated saving of 592,000 years of life (considering only premature deaths prevented). The power of rational spending on health to maximise gain is shown from a study of re-allocating those funds to the most cost-effective interventions. The change could double the benefit and save an additional 638,000 life years for the same spend [52].
It is for this reason that many countries set a threshold Incremental Cost Effectiveness Ratio (ICER) above which they are unlikely to recommend reimbursement [53]. While the best-known institution for managing these assessments may be the United Kingdom National Institute for health and Clinical Excellence (NICE), there are similar systems with budget impact or formal cost-effectiveness approval for novel treatments in many countries. By 1999, economic assessments of novel treatments were routine in the UK, Australia, Belgium, Canada, Denmark, Finland, German, Hungary, the Netherlands, Norway, Portugal, and Sweden and more have followed the fist wave in subsequent years [54, 55]. Of three OECD English-speaking countries, the UK, the USA and Canada, it is instructive to see in Fig. 4 that a focus on formal cost-effectiveness approval is associated with a slower rise in healthcare costs from1960 to 2008, and that in Table 2, that life expectancy is not proportional to health spending. At the same time, cancer death rates continue to fall in each country [56–59] (Fig. 4, Table 2).
Expenditure on health in US Dollar purchasing power parity equivalents over time for 3 English-speaking countries. The UK and Canada have explicit rationing of health expenditure for novel treatments while the USA does not. Data from http://www.oecd.org/document/16/0,3746,en_2649_37407_2085200_1_1_1_37407,00.html. Accessed 6 March 2011
The WHO has suggested that countries might decide on a threshold at a multiple of the nations’ wealth, measured by the Gross Domestic Product (GDP). Ratios of up to three times GDP have been proposed above which the resource could probably be spent better to save more lives or disability, and funding could be withheld [60]. In practice, health economic institutions seem unwilling to publish absolute ICER threshold limits, but by studying the patterns or approval or by reading discussion papers, a range of ICER levels can be established [46]. The UK NICE ICER threshold appears to be £30,000/QALY for general health and up to £45,000/QALY in the terminal phase of illness. The Netherlands has proposed €50,000–80,000/QALY for curative treatment. Sweden has levels at about 500,000 SEK/QALY. The range then seems to lie for these countries in the $45,000 to $110,000 equivalent range; or approximately two to three times per capita GDP which is in line with the WHO guidance.
While much critical publicity surrounds negative reimbursement decisions by the UK NICE, it is instructive to see how this might impact cancer patients and their physicians. Up to August 2008, NICE had approved 97% of treatments [61]. Only 3% were rejected outright while 25% were approved only for restricted subsets of patients in whom there was a higher probability of benefit and 72% were fully approved. Of 11 cancer drugs rejected for reimbursement by NICE, only 3 of 11 had proven overall survival benefit. The absolute benefit for the three were 1.8, 3.6, 4.7 months for an ICER of between 47,000 and 94,000 lb/QALY (70,000 and 141,000 dollars/QALY). Of the 9 of 11 drugs rejected with no proven survival advantage, the ICER ranged from >€30,000–171,000/QALY (>$45,000–257,000/QALY) [62]. Examples of recent positive NICE approvals include cetuximab for colorectal cancer with an ICER between £26,700 and £33,300, and Rituximab for Chronic Lymphatic Leukaemia at probably less than £30,000.
The near universal acceptance of cost/QALY assessments for new medicines has delivered a clear mechanism for cost control to most health systems. However wealthy the country may be, new treatments will be judged on the cost-effectiveness of the intervention. This is likely to be the greatest driver for the choice of health investment in the short and medium term in countries and health systems that wish to manage costs.
The magnitude of the problem—interventions that can be implemented at a local or individual physician level
Faced with the twin threats of demographics and rising treatment costs, there is now a duty for oncologists to be economically literate as well as compassionate and medically competent [63]. While many of the intrinsic problems of drug pricing and health prevention measures seem beyond the reach of individual physicians or hospitals to influence, there are practical things that can still be done which are actively promoted to physicians.
Individual physicians or hospitals can improve cost effectiveness with a program of evidence-based medicine [64–67]. For example, it has been estimated that about 30% of the total annual US expenditure on healthcare is spent on ineffective or redundant care [68].
Doctors may find it difficult to discuss money issues in clinic with patients and their families [69]. Furthermore, we are uncertain how a patient faced with a cancer diagnosis will receive and use any information on costs [70]. Despite these difficulties, the attitude of doctors to the problem of costs is changing. In 2006, a survey of Massachusetts’s oncologists shows that 88% of oncologists thought that cost should not impact their treatment decisions at all [71]. However, by 2008, opinions were changing. When asked “has your consideration of drug costs in clinical decision making changed from a year ago?” 57% reported they considered costs more and only 3% less [72].
With the help of local pharmacists, individual physicians or hospitals can save on costs of established treatment programs with a policy of bulk buying with negotiated discounts and generic substitutions [73]. Bulk discounts of more than 50% over the list price are recorded in the UK [74]. Generic substitution offers further cost-effective savings for individuals, hospitals, and health plans to exploit. The US Congressional Budget Office (CBO) looked at potential drug savings in Medicare Part D, which provides outpatient prescription drug benefits for senior citizens and people with disabilities. They found that the use of generic medications rather than brand-name medications saved beneficiaries and the program about $33 billion in 2007 [75]. They predicted an additional $14 billion in savings is expected as first-time generics entered the market in 2012.
In the US, each 1% increase in generic prescribing reduces drug costs by $1.32 billion annually [76]. Generic substitution is highly cost effective. In the UK, the average cost of a generic is a quarter of the original brand (£4.83 and £19.33 respectively) [77]. While generic substitution is one area where individual physicians and hospitals could impact quickly and significantly on the cost-effectiveness of cancer care, there is a significant variation in the practice. Only 4 of 22 countries in the EU manage to prescribe generics in two-thirds or more of prescriptions by volume [78]. To demonstrate the magnitude of cost savings that could be realized, the generic substitution of just the top 10 prescribed drugs in different EU countries could release savings of 21 to 48% to reinvest in improved patient care. Savings of 40-50% of the budget were predicted for Denmark, Germany, Portugal, Belgium and the Netherlands; of 30–40% in France, Spain, the UK and Italy; and of 20–30% in Austria and Poland.
While for many, the practice of “grey importing” of pharmaceuticals from another country seems of dubious merit, this has the potential for significant savings and is practiced widely. At present, to secure a stable price for forward planning and supply, pharmaceutical companies sell drugs at different negotiated prices in different countries. A London School of Economics study by Panos Kanavos estimates that with an open EU markets for generics, significant savings would follow. For a country that spends about 15 billion euros on medicines, and about 5 or 6 billion euros of that on generics, it could save about a quarter of that, simply because they could be paying too much. This implies savings of 1 to 1.5 billion euros, up to 10% of the entire drug budget [79].
Generic substitution is not possible with biologic drugs. This is important for each hospital to consider; however, as biological therapies are a key driver of increased cancer costs. Not only is the use of biologics growing at twice the rate of prescription drugs, but also their costs are significantly higher than conventional small molecule agents [80]. In the US alone, for 2010, it has been estimated that biologic drug sales would exceed $60 billion [81].
With the help of local pharmacists, individual physicians or hospitals can save on costs of established treatment programs with a policy of biologic drug equivalent substitutions. For biologic drugs, there is a new class of “biosimilar” or “follow-on biologic” drugs available to replace biologic drugs that have expired their patents. The first of this novel class of drug was somatrophin, licensed by the EMEA in 2006. As with all recombinant products, the tertiary structure and activity of these drugs can be altered by different growth conditions and media. All biologic drugs show some batch-to-batch variation, whether originator or biosimilar [82, 83].
A study in Nature Biotechnology in 2011 sampled three different originator biologic drugs over 2007–2011 and showed that all three drugs had detectable changes in some aspect of tertiary structures over that period when samples with different expiry dates were tested [82]. It is clear therefore that all biologic drugs may to some extent be a “biosimilar” of their original culture and preparation techniques. The degree of acceptable variation that does not change the clinical effects of the biologic drug is however the subject of great debate. This is one reason why the pathway to approval of biosimilar drugs is much more complex and prolonged that for small molecule generic drugs. This also explains why the EMEA biosimilar registration pathway, to date, has required a clinical trial with some demonstration of comparative clinical effectiveness of the “biosimilar” compared to the originator drug [83].
Biologic equivalent drugs are a challenge to make as variations in manufacturing steps of culture, extraction and purification can alter dimerisation, deamidation, oxidation and glycosylation patterns and the tertiary protein structure, and thus drug activity and toxicity. This was demonstrated in practice when an originator recombinant epoetin alfa production line was moved from the US to Europe, and immunogenicity of the new version provoked an increase in pure red cell aplasia [84]. Pure red cell aplasia is a clinical problem for the whole class of epoetin drugs when given long term for the anaemia of chronic renal failure, but a significant rise in cases was detected. As a consequence, by 2008, fifteen countries have banned automatic substitution of biosimilars [85].
Biosimilar is a regulatory term created by the European Medicines Agency (EMEA), to denote a tested and regulated drug with the same expected sequence, safety and activity as the originator reference drug [86]. In the US the term is “biologic follow-on drug” to denote such drugs regulated and licensed by the US Food and Drugs administration (FDA). These share the same DNA sequence as the originator product, and have to demonstrate equivalent activity and toxicity before approval with clinical trials and agreed post-marketing surveillance. The EMEA explicitly recognizes that existing biosimilars can and should have the same International Non-Proprietary Name (INN) as their reference product.
Physicians need to be aware that this is very different to simple unregulated and unlicensed copy biologic drugs that have been produced prior to patent expiry and marketed outside the EU and North America. The EMEA requires significantly more testing for biosimilars than for small molecule generics, including clinical trials and a formal post-marketing surveillance program before approval.
In Asia, the term “biogeneric” has been used to describe unregulated and unlicensed copy biologic drugs that have been produced prior to patent expiry. A recent review from India, reported more than 50 brands now on sale and that price becomes the key marketing force [87]. Biogeneric copy drugs appear within 2 to 3 years of market launch, forcing down prices for both biogeneric producers and innovator companies. This price-sensitivity has led to companies cutting quality controls with the result that significant differences in comparability with the innovator drug are found, which lead to very different dose and activity profiles.
In contrast to EMEA-regulated biosimilars, unregulated biogeneric copy drugs of biologics may share the same DNA sequence, but have potentially significant variations in post-translation chemistry; seen as novel isoforms of the drugs with altered glycosylation patterns and significantly altered activity [88]. Many will never have had formal clinical testing. Within the EU however, a novel regulatory pathway and licensing system has been introduced to ensure that biosimilars behave in the same way as originator drugs [89]. Confusion about the difference between unregulated copy biologics and regulated biosimilar or biologic follow-on equivalent drugs will need to be clarified for oncologists to be confident to initiate a program of biosimilar equivalent substitution in their own practice [85].
For 2011, the EU has only a limited range of biosimilar drug classes approved for oncology. To date these are all supportive care cancer hormonal agents; biosimilar erythropoetins and biosimilar filgastrim G-CSF. With EU patent time expiry, however, this will soon include the more structurally complex monoclonal antibody (mAb) therapies such as imatinib for CML, trastuzumab for HER2- overexpressing breast cancer, and rituximab for B-cell lymphoma. The market for these therapies is estimated at $36.4 to $40 billion [90, 91]. In preparation for those, the EMEA released draft guidelines for their biosimilar development in November 2011. Estimated by patent expiry dates, oncology antibody products will appear in 2015, followed by darbepoetin biosimilars in 2016 [92].
To succeed, biosimilar drugs will need to be cheaper than their original reference drugs. In the UK, the Scottish National Health Service has published data on the cost saving from buying biosimilar G-CSF. Based on its use in myelotoxic breast cancer chemotherapy, the estimated savings over a 12-week schedule was more than £300 per patient.
Annual savings of €1.6 billion per year have been predicted if the European Union could realize a 20% price reduction of just five patent expired biopharmaceuticals [93]. Predictions are that cost savings of 25 to 30% can be achieved [94]. Looking forward further to 2020, there are 20 biologic drugs in the EU which will come off patent that generate more than $300 million in revenue in Europe alone [95]. Such savings will be hard to resist in such a regulated EU health market. If biosimilar drugs escape immunogenicity and safety scares, then they are likely to become closely equated to “generics” for small molecule medicines. Few oncologists are likely to be able to name the producers of the major off-patent cytotoxic drugs at their hospitals and clinics. Even less will have ever been involved in their selection and purchase. With the pressure for cost control, it seems inevitable that they will come under automatic promotion by the health technology appraisal systems common in European health systems such as NICE. With such promotion, the global use of biosimilars is predicted to follow a compound annual growth rate of 65.8% per year [96].
The country that has set the benchmark for early adoption of biosimilars in Europe is Germany. Even in early 2008, the half of epoetin (55%) and a third of GCSF (31%) prescriptions were biosimilars [97]. The effect of price competition on biologic drug use was also illustrated by epoetin in Germany. Three biosimilars were introduced in 2008 with a 30% below the originator price. A price reduction by the originator was followed by further price reductions by the biosimilars and then again by one of the originators [98].
The contrast between the EU and the US is in the speed and clarity of the legal development of regulator pathways for biosimilar drug development. The issue appears to be politicized in the USA where the “Biologics Price Competition and Innovation Act” is in discussion. In recent US government debates, Time magazine suggested that in the first six months of 2009 alone, drug and biotech companies and their trade associations spent more than $110 million lobbying to influence decisions with more than 2 lobbyists for every member of Congress [99]. Once an effective American legal pathway to biosimilar introduction is present, sales are also likely to rise rapidly. This is predicted by the high price of originator biologics and patient pressure because so many patients have to pay significant parts of medical bills through co-insurance fees. This is seen already in the American generic market, where such drugs account for more than 50% of the total US prescriptions [100]. For the US, patents have already expired on biologic drugs representing more than $15 billion in costs annually [101]. Even with modest price reductions of 20–30%, this represents a cost saving of $3 to $4.5 billion that will be impossible for health insurers and hospitals to resist. A 2008 analysis by Robert Shapiro suggested that generic versions of the top 12 categories of biologics whose patents have expired or will expire soon could save Americans up to $108 billion in the first 10 years and as much as $378 billion over two decades.
The impression that a biosimilar-regulated USA will embrace biosimilars quickly is supported by predictions that biosimilar epoetin market gain is likely to be more rapid and more complete in the United States compared to biosimilar erosion seen in Europe [102]. More than half of surveyed US physicians reported that they would begin prescribing a biosimilar epoetin within 6 months of its launch and 88% expect to be using it within a year. In contrast, the majority of surveyed German nephrologists did not begin using biosimilar epoetin until the drugs had been on the market for 13–24 months. Uptake of biosimilars among surveyed French nephrologists has been even more conservative—60% of surveyed physicians report they still do not prescribe biosimilar epoetin [103].
Conclusions
Without intervention, even wealthy countries face a crisis in healthcare spending, driven principally by novel high-cost biologic cancer drug development and to a lesser extent by demographics. This places a responsibility upon oncologists to become more economically literate, with the confidence to read and react to new cost-effectiveness studies.
While individual hospitals and physicians may seem powerless to make a difference, a local program of evidence-based medicine, generic, and biosimilar substitution could help ensure healthcare costs remain sustainable. Biosimilar drug substitution offers the latest way for oncologists to save costs for reinvestment. However, as biosimilar medicines represent a whole new class of medicines, this step will be more complex than generic substitution. It may require a significant program of education and partnership between pharmacists, physicians, and patients to be realized fully.
For centralized healthcare systems, and those with a strong tradition of generic medicine use, biosimilar use will clearly rise with predictions of more than 80% of prescriptions of some biologic drugs within 1 year of market entry in the USA. Delaying the implementation of such programs, however, risks a real crisis in healthcare delivery for many countries and hospitals that few can now afford.
References
Parkin DM (2001) Global cancer statistics in the year 2000. Lancet Oncol 2(9):533–43
Mulcahy N (2009) Time to consider cost in evaluating cancer drugs in United States? Medscape Medical News July 14. http://www.medscape.com/viewarticle/705689
Behnke N, Hueltenschmidt N, Pasternak A, Singh K (2011) Biosimilars: a marathon, not a sprint. Industry Brief 12/16/09. http://www.bain.com/bainweb/publications/publications_detail.asp?id=27518&menu_url=publications_results.asp
Medicare Payment Advisory Commission: report to the Congress: Medicare Payment Policy, (3, 2010) (Washington, DC). Available at http://www.medpac.gov/documents/Mar10_EntireReport.pdf. Accessed 3 March 2011
Medical Subject Headings (MeSH) portal of the USA National Library of Congress PubMed database. Available at http://www.ncbi.nlm.nih.gov/mesh
Google “related sites” programme. Available at http://www.google.com/help/features.htmlwebportal
Schwartz K (2010) Projected costs of chronic diseases. Health Care Cost Monitor. January 22
WHO (2008) Cancer to be world’s top killer by 2010. USA Today, 12/9/2008. Available from http://www.usatoday.com/news/health/2008-12-09-cancer_N.htm. Accessed 13 March 2011
Ibrahim E, Bin SB, Banjar L et al (2008) Current and future cancer burden in Saudi Arabia: meeting the challenge. Hematol Oncol Stem Cell Ther 1(4):210–5
The Rising Cost of Cancer Care: Who Takes Responsibility? J Multidisciplinary Cancer Care. October 2010, Vol 3, no 7. Available at http://www.jomcc.com/article/rising-cost-cancer-care-who-takesresponsibility. Accessed 13 march 2011
Cost of US healthcare (1980) US News and World Report Magazine. Sept 1st
Centers for Medicare and Medicaid Services, Office of the Actuary, National Health Statistics Group, Historical; NHE summary including share of GDP, CY 1960–2007. http://www.cms.hhs.gov/NationalHealthExpendData/(see Historical; NHE summary including share of GDP, CY 1960–2007; file nhegdp07.zip). Accessed 22 Feb 2011
Goldman DP, McGlynn EA (2005) US Health Care—Facts about cost, access, and quality. The RAND Corporation 2005. Available at http://www.rand.org/pubs/corporate_pubs/2005/RAND_CP484.1.pdf. Accessed 13 March 2011
Ward E, Halpern M, Schrag N et al (2008) Association of insurance with cancer care utilization and outcomes. CA Cancer J 58:9–31
Nelson R (2011) Medicare unable to control rising costs of cancer care. Medscape Medical News. Available at http://www.medscape.com/viewarticle/587974. Accessed 13 march 2011
Auguste BG, Laboissière M, Mendonca LT (2009) How health care costs contribute to income disparity in the United States. McKinsey Quarterly (April)
Noah T (2011) Health haves and have- nots. The true cost of spiraling medical inflation. Slate Magazine. http://www.slate.com/id/2216431/. Accessed 6 March 2011
http://www.mckinseyquarterly.com/How_health_care_costs_contribute_to_income_disparity_in_the_United_States_2328. Accessed 6 March 2011
Employer Health Benefits 2009 Annual Survey, Kaiser Family Foundation and Health Research Educational Trust
Cavallo J (2011) Containing the high cost of cancer care. Four experts examine the issue and offer solutions. The ASCO Post, February 15, 2011, Volume 2, Issue 3
Himmelstein DU, Thorne D, Warren E et al (2009) Medical bankruptcy in the United States, 2007: results of a national study. Am J Med 122:741–6
Malin JL (2010) Wrestling with the high price of cancer care: should we control costs by individuals’ ability to pay or society’s willingness to pay? J Clin Oncol 28(20):3212–4
Keehan S, Sisko A, Truffer C, et al. (2008) Health spending projections through 2017: the baby-boom generation is coming to medicare. Health Aff March 27: w145–w55; published ahead of print February 26. doi:10.1377/hlthaff.27.2.w145. Accessed 22 Feb 2011
Chernew ME, Hirth RA, Cutler DM (2009) Increased spending on health care: longterm implications for the Nation. Health Aff 28(5):1253–5
WHO—Spectre of ageing population worries economistsBulletin of the World Health Organization 88(3). Available at http://www.who.int/bulletin/volumes/88/3/10-030310/en/index.html
Schickedanz A (2010) Of value: a discussion of cost, communication, and evidence to improve cancer care. Oncologist 15(Suppl 1):73–9
Adams CP, Brantner VV (2006) Estimating the cost of new drug development: is it really 802 million dollars? Health Aff (Millwood) 25:420–8
Rucker NL (2007) Biologics in perspective: expanded clinical options amid greater cost scrutiny. AARP (online)
Hillner BE, Smith TJ (2009) Efficacy does not necessarily translate to cost effectiveness: a case study in the challenges associated with 21st-century cancer drug pricing. J Clin Oncol 27:2111–3
Perrin S (2010) Therapeutic decision making in oncology. Hosp Pharm Eur 52:36–7
Sarin R (2008) Criteria for deciding costeffectiveness for expensive new anticancer agents. J Can Res Ther 4:1–2
Bach P (2009) Limits on medicare’s ability to control rising spending on cancer drugs. N Engl J Med 360:626–33
Fojo T, Grady C (2009) How much is life worth: cetuximab, non-small cell lung cancer, and the $440 billion question. J Natl Cancer Inst 101(15):1044–8
“Is the price of cancer treatment falling?” Paper presented at the annual meeting of the economics of population health: Inaugural Conference of the American Society of Health Economists, TBA, Madison, WI, USA, Jun 04, 2006. Available http://www.allacademic.com/meta/p93517_index.html, Accessed 12 March 2011
Schrag D (2004) NEJM 351:317–9
Jansman FGA, Maarten J, Postma MJ, Brouwers JRBJ (2007) PharmacoEconomics 25(7):537–62
Uyl-de Groot CA, de Groot S, Steenhoek A (2010) The economics of improved cancer survival rates: better outcomes, higher costs. Expert Rev Pharmacoeconomics Outcomes Res 10(3):283–92
Wilking N, Jönsson B (2011) A pan- European comparison regarding patient access to cancer drugs. Available at http://ki.se/content/1/c4/33/16/Cancer_Report.pdf. Accessed 13 March 2011
US National Cancer Institute website for cancer prevalence and cost of care projection data. Available at http://costprojections.cancer.gov/graph.php. Accessed 3 March 2011
Cancer Network (2011) NIH study predicts the cost of cancer care will increase 27% by 2020. Cancer Network, January 12. http://www.cancernetwork.com/practice/content/article/10165/1776564
The relative effect of demographics and increased unit treatment costs on the spending on Medicare, Medicaid and Social Security as a% of USA GDP. Office of management and budget. www.whitehouse/omb. Accessed 22 Feb 2011
Fleck L (2006) The costs of caring: Who pays? Who profits? Who panders? Hastings Cent Rep 36(3):13–7
Murray CJL, Evans DB, Acharya A et al (2000) Development of WHO guidelines on generalized cost-effectiveness analysis. Health Econ 9:235–251
Romond EH, Perez EA, Bryant J et al (2005) Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 353:1673–84
Garrison LP, Perez EA, Dueck A et al (2006) Cost-effectiveness analysis of trastuzumab in the adjuvant setting for treatment of HER2+ breast cancer. J Clin Oncol 24(18S):6023
Kirkdale R, Krell J, Brown CO et al (2010) The cost of a QALY. QJM 103(9):715–20
Newby LK, Eisenstein EL, Califf RM et al (2000) Cost effectiveness of early discharge after uncomplicated acute myocardial infarction. N Engl J Med 342(11):749–55
Rawlins MD, Culyer AJ (2004) National Institute for clinical excellence and its value judgments. BMJ 329:224. doi:10.1136/bmj.329.7459.224, (Published 22 July 2004). Accessed 13 march 2011
WHO (2000) The World Health Report 2000
Tengs TO (1997) Dying too soon: how costeffectiveness analysis can save lives. Irvine, California, University of California, National Center for Policy Analysis (Policy Report no 204)
Huttin C (1999) Drug price divergence in Europe: regulatory aspects. Health Aff 18:245–9
McCabe C, Claxton K, Culyer AJ (2008) The NICE cost-effectiveness threshold: what it is and what that means. Pharmacoeconomics 26(9):733–44
Cancer Trends Progress Report – 2009/2010 Update, National Cancer Institute, NIH, DHHS, Bethesda, MD, April 2010, http://progressreport.cancer.gov. Accessed 13 March 2011
Faden RR, Chalkidou K, Appleby J et al (2009) Expensive cancer drugs: a comparison between the United States and the United Kingdom. Milbank Quarterly 87:789–819. doi:10.1111/j.1468-0009.2009.00579.x
Malvezzi M, Arfé A, Bertuccio P et al (2011) European cancer mortality predictions for the year 2011. Ann Oncol (first published online). doi:10.1093/annonc/mdq774
Marrett LD, De P, Airia P et al (2008) Cancer in Canada in 2008. CMAJ 179(11):1163–70
Davis K, Schoen C, Stremkis K (2010) Mirror, mirror on the wall: how the performance of the US Health Care System Compares Internationally,MPP Commonwealth fund, June 23. Available at http://www.commonwealthfund.org/Content/Publications/Fund-Reports/2010/Jun/Mirror-Mirror-Update.aspx. Accessed 13 March 2011.
Cornes P (2008) Cost-effectiveness of treating cancer anaemia. In: Nowrousian MR (ed) Recombinant human erythropoietin (rhEPO) in Clinical Oncology—Scientific and clinical aspects of anemia in cancer. 2nd Edition, Springer-Verlag, Chapter 34, pp 813–49
Presentation by Andrew Dillon, the head of the National Institute for Clinical Excellence (NICE), the UK’s technology assessment agency August 04, 2008. Slides on file
Raftery J (2009) NICE and the challenge of cancer drugs. BMJ 338:b67
Andrews G, Simonella L, Lapsley H et al (2006) Evidence-based medicine is affordable: the cost-effectiveness of current compared with optimal treatment in rheumatoid and osteoarthritis. J Rheumatol 33(4):671–80
Elzawawy A (2009) The “Win-Win” initiative: a global, scientifically based approach to resource sparing treatment for systemic breast cancer therapy. World J Surg Oncol 7:44. doi:10.1186/1477-7819-7-44
Hoverman JR, Russell S (2011) Responsible spending in cancer care: the steps that really matter. The ASCO post. January 15, Volume 2, Issue 2. Available at http://www.ascopost.com/articles/january-15-2011/responsible-spending-in-cancercare-the-steps-that-really-matter. Accessed 14 march 2011
Meropol NJ, Schrag D, Smith TJ et al (2009) American Society of Clinical Oncology guidance statement: the cost of cancer care. J Clin Oncol 27:3868–74
How providers can lower costs and improve patient care using evidence-based medicine. An oracle white paper July 2009. Available at http://www.oracle.com/us/industries/018896.pdf. Accessed 13 March 2011
US Senate Finance Committee Roundtable Proceedings on Healthcare Delivery System Reform, Allan Korn, M.D., Senior Vice President and Chief Medical Officer Blue Cross and Blue Shield Association, April 2009
Schrag D, Hanger M (2007) Medical oncologists’ views on communicating with patients about chemotherapy costs: a pilot survey. J Clin Oncol 25:233–7
Mileshkin L, Schofield PE, Jefford M et al (2009) To tell or not to tell: the community wants to know about expensive anticancer drugs as a potential treatment option. J Clin Oncol 27:5830–7
Nadler E, Eckert B, Neumann PJ (2006) Do oncologists believe new cancer drugs offer good value? Oncologist 11:90–5
Cote B (2009) Oncologists: Cost-Effectiveness Policy Necessary Despite Quality of Care Warnings. Oncology Business Review. ONCBIZ.COM, September, p. 26. http://www.oncbiz.com/documents/OBR_SEPT_MRR.pdf
de Joncheere K, Rietveld AH, Huttin C (2002) Experiences with generics. Int J Risk Safety Med 15:101–19
Kanavos P (2007) Do generics offer significant savings to the UK National Health Service? Curr Med Res Opin 23(1):105–16
Generic drugs in USA’s Medicare Part D saved beneficiaries and program $33 billion in 2007, says new CBO study. http://www.thepharmaletter.com/file/98437/generic-drugs-in-usas-medicare-part-d-saved-beneficiaries-and-program-33-billion-in-2007-says-new-cbo-study.html. Accessed 22 Feb 2011.
Privitera MD (2008) Generic antiepileptic drugs: current controversies and future directions. Epilepsy Curr 8:113–7
Market key facts. www.britishgenerics.co.uk/marketkeyfacts.htm
Data from the European Generic Medicines Association. http://www.egagenerics.com/doc/fac-GxMktEur_2006.pdf
Wagstaff A (2007) No-name heroes can save Europe billions. Cancerworld March–April: 24–28. Available at http://www.cancerworld.org/pdf/8503_CW17_24-28_DrugWatch.pdf
Grabowski H, Cockburn I, Long G (2006) The market for follow-on biologics: how will it evolve? Follow-on biologics. Health Aff 25(5):1291–1301
Schiestl M, Stangler T, Torella C, Cepeljnik T, Toll H, Grau R (2011) Acceptable changes in quality attributes of glycosylated biopharmaceuticals. Nat Biotechnol 29(4):310–2
Mellstedt H, Niederwieser D, Ludwig H (2008) The challenge of biosimilars. Ann Oncol 19:411–9
Nowicki M (2007) Basic facts about biosimilars. Kidney Blood Press Res 30:267–72
Giezen TJ, Mantel-Teeuwisse AK, Straus SM et al (2008) Safety-related regulatory actions for biologicals approved in the United States and the European Union. JAMA 300:1887–96
Kane A (2008) Fifteen countries had banned automatic substitution of biosimilars – EGA. APM Health Europe. http://health.apmnews.com/publicsearch.php?mots=Kane&depsPage=26
Schellekens H (2009) Biosimilar therapeutics—what do we need to consider? NDT Plus 2(Supp l1):i27–i36. doi:10.1093/ndtplus/sfn177
Mody R, Goradia V, Gupta D (2011) How similar are biosimilars in India? A blind comparative study. Pharmafocusasia. Available at http://www.pharmafocusasia.com/research_development/blind-comparative-study.html. Accessed 13 March 2011
Schellekens H (2004) Biosimilar epoetins: how similar are they? Eu J Hosp Pharm 3(8–12):81
Arnett SO, Teillaud JL, Wurch T, et al (2011) IBC’s 21st Annual Antibody Engineering and 8th Annual Antibody Therapeutics International Conferences and 2010 Annual Meeting of The Antibody Society December 5–9, San Diego, CA USA. mAbs 3(2):135–155
Rossert J (2007) EMEA Guidelines on biosimilars and their clinical implications. Kidney Blood Press Res 30(Suppl 1):13–17
Sinha G (2011) EU mAb biosimilars path. Nat Biotechnol 29:10. doi:10.1038/nbt0111-10b, Published online 10 January 2011. Accessed 13 March 2011
The future of pharmaceuticals: generic medicines enhancing pharmaceutical competition and ensuring healthcare sustainability. Available at http://www.egagenerics.com/doc/ega_FuturePharmaceuticals.pdf. Accessed 13 March 2011
Jelkmann W (2010) Biosimilar epoetins and other “follow-on” biologics: update on the European experiences. Am J Hematol 85:771–80. doi:10.1002/ajh.21805
Tolve A (2010) Forecasting the potential of the biosimilars market. Sep 27, http://social.eyeforpharma.com/story/forecastingpotential-biosimilars-market. Accessed 13 March 2011
The future of biosimilars—Market forecasts to 2015. Dec 2009. http://www.docstoc.com/docs/64933502/The-Future-of-Biosimilars—Market-Forecasts-to-2015-Opportunity-Analysis-and-Regulatory-Pathways. Accessed 13 March 2011
Sheppard A (2010) The biosimilars market today and tomorrow. Pharm Technol Eur Volume 22, Issue 9, Sep 1. http://pharmtech.findpharma.com/pharmtech/Manufacturing/The-Biosimilars-Market-Today-And-Tomorrow/ArticleStandard/Article/detail/685049. Accessed 13 march 2011
Mattison N, Mestre-Ferrandiz J, Towse A (eds) (2010) Biosimilars: How much entry and price competition will result? Office of Health Economics (OHE), London
Tumulty K, Scherer M (2009) How drug industry lobbyists won on health-care. Time. Thursday, Oct 22
Morrison AJ (2007) Biosimilars in the United States: a brief look at where we are and the road ahead. Biotech Law Rep 26:463–8
Grabowski H, Ridley DB, Schulman KA (2007) Entry and competition in generic biologics. Manag Decis Econ 28:439–51
Decision Resources (2011) Biosimilar erosion of branded esa market share will be more rapid in the US than in Europe. http://decisionresources.com/News-and-Events/Press-Releases/BAS-11410-(1). Accessed 13 March 2011
Klusacek ME, Malecki M (2010) Biosimilars advisory service: physician perspectives on erythropoietin stimulating agents, insulin, and human growth hormone. November. http://decisionresources.com/Products-and-Services/Report?r=basxpr0610. Accessed 13 March 2011
Conflict of interest statement
The author does not have any conflict of interest to declare.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Cornes, P. The economic pressures for biosimilar drug use in cancer medicine. Targ Oncol 7 (Suppl 1), 57–67 (2012). https://doi.org/10.1007/s11523-011-0196-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-011-0196-3