Abstract
Over the past decades, there has been extensive study on the design of porous bioceramic scaffolds with controlled bioactivity and biodegradation in bone tissue repair. A variety of suggestive models and concepts have been proposed with regard to the role of microstructure and composition of biomaterials which affect new bone tissue growth. However, it is a challenge to fabricate functional scaffolds with the desired physiological properties and osteogenic potentials that is comparable to the bone’s natural healing time scale. We demonstrate a one-step versatile fabrication of a single-phase and homogenously mixed bioactive load-bearing scaffolds (Sr-CS, CaSiO3/Ca2SiO4, and CaP) with superior biological properties in a critical size bone defect (Ø ~ 6.0 × 8.0 mm). In vivo study revealed the CaSiO3/Ca2SiO4 scaffold had the best amount of new bone growth and osteogenic repair. The Sr-CS exhibited an adequate pore network for rapid inorganic exchange and moderate mechanical stability; however, the CaSiO3/Ca2SiO4 saw over-fast resorption and mass loss compared to the Sr-CS and CaP. On the other hand, the CaP scaffold saw mechanically outstanding elastroplastine and stability but had limited biodegradation of its constructs which retarded new cancellous bone growth. The CaSiO3/Ca2SiO4 group saw superior acceleration and formation of mineralized new bone tissues in the defect. Moreover, the CaSiO3/Ca2SiO4 showed appreciable decay of the biomaterials beneficial for osteogenic cell activity. The dramatic stimulation of bone repair and angiogenesis with the CaSiO3/Ca2SiO4 suggests a promising application of this novel bioactive scaffold in the repair of skeletal defects.
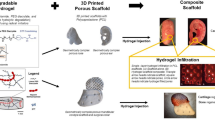
Graphical abstract
Systemic representation of the fabricated microspheres with in vivo and in vitro study analysis










Similar content being viewed by others
References
J Biomaterials Currey (2001) Sacrificial bonds heal bone. Nature 414:699
Li H, Xue K, Kong N, Liu K, Chang J (2014) Silicate bioceramics enhanced vascularization and osteogenesis through stimulating interactions between endothelia cells and bone marrow stromal cells. Biomaterials 35:3803–3818
Jones AC, Arns CH, Hutmacher DW, Milthorpe BK, Sheppard AP, Knackstedt MA (2009) The correlation of pore morphology, interconnectivity and physical properties of 3D ceramic scaffolds with bone ingrowth. Biomaterials 30:1440–1451
Boccaccini AR, Blaker JJ (2005) Bioactive composite materials for tissue engineering scaffolds. Expert Rev Med Devices 2:303–317
Zhou H, Lee J (2011) Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 7(7):2769e2781
Ha SW, Jang HL, Nam KT, Beck GR Jr (2015) Nano-hydroxyapatite modulates osteoblast lineage commitment by stimulation of DNA methylation and regulation of gene expression. Biomaterials 65:32e42
Ooms EM, Wolke JGC, van de Heuvel MT, Jeschke B, Jansen JA (2003) Histological evaluation of the bone response to calcium phosphate cement implanted in cortical bone. Biomaterials 24:989–1000
Ooms E, Wolke J, van der Waerden J, Jansen J (2002) Trabecular bone response to injectable calcium phosphate (Ca–P) cement. J Biomed Mater Res 61:9–18
Jansen J, Ooms E, Verdonschot N, Wolke J (2005) Injectable calcium phosphate cement for bone repair and implant fixation Orthop. Clin North Am 36:89–95
Chow LC (2009) Next generation calcium phosphate-based biomaterials Dent. Mater J 28:1–10
LeGeros R (2002) Properties of osteoconductive biomaterials: calcium phosphates Clin. Orthop Relat Res 395:81–98
Wu Cand Chang J (2013) A review of bioactive silicate ceramics Biomed. Mater. 8:032001
Wu C, Chang J, Zhai W, Ni S, Wang J (2006) Porous akermanite scaffolds for bone tissue engineering: preparation, characterization, and in vitro studies. J Biomed Mater Res B: Appl Biomater 78B:47–55
Ni SY, Chang J, Chou L (2006) A novel bioactive porous CaSiO3 scaffold for bone tissue engineering. J Biomed Mater Res A 76:196–205
Lin KL, ZhaiWY NSY, Chang J, Zeng Y, Qian WJ (2005) Study of themechanical property and in vitro biocompatibility of CaSiO3 ceramics. Ceram Int 31:323–326
Shie MY, Ding SJ (2013) Integrin binding and MAPK signal pathways in primary cell responses to surface chemistry of calcium silicate cements. Biomaterials 34:6589–6606
Wang C, Lin KL, Chang J, Sun J (2013) Osteogenesis and angiogenesis induced by porous b-CaSiO3/PDLGA composite scaffold via activation of AMPK/ERK1/2 and PI3K/Akt pathways. Biomaterials 34:64–77
Ni SY, Chang J (2009) In vitro degradation, bioactivity, and cytocompatibility of calcium silicate, dimagnesium silicate, and tricalcium. phosphate bioceramics. J Biomater Appl 24:139–158
Ni S, Lin K, Chang J, Chou L (2008) Beta-CaSiO3/beta-Ca3(PO4)2 composite materials for hard tissue repair: in vitro studies. J Biomed Mater Res Part A 85:72–82
Wang C, Xue Y, Lin K, Lu J, Chang J, Sun J (2012) The enhancement of bone regeneration by a combination of osteoconductivity and osteostimulation using beta-CaSiO3/beta-Ca3(PO4)2 composite bioceramics. Acta Biomater 8:350–360
Sun LA, Shao M, Yang H, Ma X, He C, Gao D, Liu Q, Yan Y, Xu S, Jianzhong S, Gou Z (2016) Outstanding mechanical response and bone regeneration capacity of Robocast dilute magnesium-doped wollastonite scaffolds in critical size bone defect. J Mater Chem B 4:3945–3958
Marie PJ, Hott M, Modrowski D, De Pollak C, Guillemain J, Deloffre P et al (1993) An uncoupling agent containing strontium prevents bone loss by depressing bone resorption and maintaining bone formation in estrogen-deficient rats. J Bone Miner Res 8:607–615
Bonnelye E, Chabadel A, Saltel F, Jurdic P (2008) Dual effect of strontium ranelate: stimulation of osteoblast differentiation and inhibition of osteoclast formation and resorption in vitro. Bone 42:129–138
Bohner M, Tadier S, van Garderen N, de Gasparo A, Deobelin N, Baroud G (2013) Synthesis of spherical calcium phosphate particles for dental and orthopedic applications. Biomatter 3:e25103–e25115
Deville S, Saiz E, Nalla RK, Tomsia AP (2006) Freezing as a path to build complex composites. Science 311:516–518
Descamps M, Duhoo T, Monchau F, Lu J, Hardouin P, Hornez JC, Leriche A (2008) Manufacture of macroporous β-tricalcium phosphate bioceramics. J Eur Ceram Soc 28:149–157
Rezwan K, Chen QZ, Blaker JJ, Boccaccini AR (2006) Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 27:3413
Bose S, Vahabzadeh S, Bandyopadhyay A (2013) Bone tissue engineering using 3D printing. Mater Today 16:496–504
Gou Z, Chang J (2004) Synthesis and in vitro bioactivity of dicalcium silicate powders. J Eur Ceram Soc 24:93–99
Ghamor-Amegavi EP, Yang X, Qiu J, Xie L, Pan Z, Wang J, Zhang X, Ke X, Zhao T, Zhang L, Gou Z (2020) Composition control in biphasic silicate microspheres on stimulating new bone regeneration and repair of osteoporotic femoral bone defect. J Biomed Mater Res B Appl Biomater 108(2):377–390
Szpalski C, Wetterau M, Barr J, Warren SM (2012) Bone tissue engineering: current strategies and techniques-part I: scaffolds. Tissue Eng Part B Rev 18:246–257
Wong TM, Lau TW, Li X, Fang C, Yeung K, Leung F (2014) Masquelet technique for treatment of posttraumatic bone defects. Sci World J 710302:2356–6140
Fu Q, Rahaman MN, Day DE (2009) Accelerated conversion of silicate bioactive glass (13–93) to hydroxyapatite in aqueous phosphate solution containing polyanions. J Am Ceram Soc 92:2870–2876
Bucking TM, Hill ER, Robertson JL, Maneas E, Plumb AA, Nikitichev DI (2017) From medical imaging data to 3D printed anatomical models. PLoS ONE 12(5):e0178540
Rengier F, Mehndiratta A, von Tengg KH, Zechmann CM, Unterhinninghofen R, Kauczor HU, Giesel FL (2010) Int J Comput Assisted Radiol Surg 5:335
Friedman T, Michalski M, Goodman TR, Brown JE (2016) Skeletal Radiol 45:307
Cubo N, Garcia M, Del Cañizo JF, Velasco D, Jorcano JL (2016) 3D bioprinting of functional human skin: production and in vivo analysis. Biofabrication 9(1):015006
Xiong S, Zhang X, Lu P, Wu Y, Wang Q, Sun H, Heng BC, Bunpetch V, Zhang S, Ouyang H (2017) Sci Rep 7:4288
Mosadegh B, Xiong G, Dunham S, Min JK (2015) Current progress in 3D printing for cardiovascular tissue engineering. Biomed Mater 10:034002
Ho CMB, Mishra A, Lin PTP, Ng SH, Yeong WY, Kim YJ, Yoon YJ (2017) Macromol Biosci 17:1600250
Mastrogiacomo M, Scaglione S, Martinetti R, Dolcini L, Beltrame F, Cancedda R, Quarto R (2006) Role of scaffold internal structure on in vivo bone formation in macroporous calcium phosphate bioceramics. Biomaterials 27:3230–3237
Kasten P, Beyen I, NiemeyerP LR, Bohner M, Richter W (2008) Porosity and pore size of β-tricalcium phosphate scaffold can influence protein production and osteogenic differentiation of human mesenchymal stem cells: an in vitro and in vivo study. Acta Biomater 4:1904–1915
von Doernberg MC, von Rechenberg B, Bohner M, Grünenfelder S, van Lenthe GH, Müller R, Gasser B, Mathys R, Baroud G, Auer J (2006) In vivo behavior of calcium phosphate scaffolds with four different pore sizes. Biomaterials 27:5186–5198
Wang H, Zhi W, Lu X, Li X, Duan K, Duan R, Mu Y, Weng J (2013) Comparative studies on ectopic bone formation in porous hydroxyapatite scaffolds with complementary pore structures. Acta Biomater 9(9):8413–8421
Ke X, Zhuang C, Yang X, Fu J, Xu S, Xie L, Gou Z, Wang J, Zhang L, Yang G (2017) Enhancing the osteogenic capability of core-shell bilayered bioceramic microspheres with adjustable biodegradation. ACS Appl Mater Interfaces 9(29):24497–24510
Wang C, Lin K, Chang J, andSun J, (2014) The stimulation of osteogenic differentiation of mesenchymal stem cells and vascular endothelial growth factor secretion of endothelial cells by b-CaSiO3/b-Ca3(PO4)2 scaffolds. J Biomed Mater Res Part A 102:2096–2104
Wu C, Chen Z, Yi D, Chang J, Xiao Y (2014) Multidirectional effects of Sr-, Mg-, and Si-containing bioceramic coatings with high bonding strength on inflammation, osteoclastogenesis, and osteogenesis. ACS Appl Mater Interfaces 6:4264–4276
De Aza PN, García-Bernal D, Cragnolini F, Velasquez P, Meseguer-Olmo L (2013) The effects of Ca2SiO4–Ca3(PO4)2 ceramics on adult human mesenchymal stem cell viability, adhesion, proliferation, differentiation and function. J Mater Sci Eng C Mater Biol Appl 33:4009–4020
Funding
This work was supported by the National Key Research and Development Program of China (2018YC1105401) and the Zhejiang Provincial Natural Science Foundation of China (LY15H180006).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cong, Y., Liang, Z., Jianping, N. et al. Rational design and fabrication of monophasic bioceramic microspheres with enhanced mechanical and biological performances in reconstruction of segmental bone defect. Med Biol Eng Comput 60, 1691–1703 (2022). https://doi.org/10.1007/s11517-022-02571-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-022-02571-7