Abstract
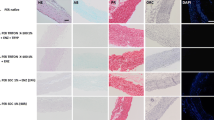
Decellularized pericardial tissue is a strong candidate for a TEHV material as ECM is present to guide cellular infiltration and fixed porcine and bovine pericardial tissue have existing use in bioprosthetic heart valves. In this work, we compare the mechanical and microstructural properties of decellularized-sterilized (DS) porcine, bovine, and bison pericardial tissues with respect to use as a TEHV. H&E staining was used to verify removal of cellular content post-decellularization and to evaluate collagen fiber structure. Additionally, uniaxial and biaxial tension testing were used to compare mechanical performance and, for the latter, acquire constitutive model parameters for subsequent finite element (FE) modeling. H&E staining revealed complete removal of cellular content and good collagen fiber structure. Tensile testing showed comparable mechanical strength between the three DS pericardial tissues and considerably stronger mechanical properties compared to native tissues. Bovine and bison DS pericardial tissues showed the strongest mechanical performance in the FE models with bison demonstrating the overall best mechanical characteristics. The increased thickness of bovine and bison tissues coupled with the strong mechanical behavior and ECM structure indicates that these materials will be resistant to damage until sufficient cellular infiltration has occurred such that damaged tissue can be repaired.
Graphical abstract








taken from sites in the FE models at the top of the commissures and leaflet belly at set points in the cardiac cycle. Data is shown for DS porcine, bovine and bison pericardium, and native porcine aortic valve

Similar content being viewed by others
References
d’Arcy JL, Prendergast BD, Chambers JB, Ray SG, Bridgewater B (2010) Valvular heart disease: the next cardiac epidemic. Heart 97:91–93. https://doi.org/10.1136/hrt.2010.205096
Piazza N, Bleiziffer S, Brockmann G, Hendrick R, Deutsch MA, Opitz A, Mazzitelli D, Tassani-Prell P, Schreiber C, Lange R (2011) Transcatheter Aortic Valve Implantation for Failing Surgical Aortic Bioprosthetic Valve: From Concept to Clinical Application and Evaluation (Part 1). JACC Cardiovasc Interv 4:721–732. https://doi.org/10.1016/j.jcin.2011.03.016
Leopold JA (2012) Cellular mechanisms of aortic valve calcification. Circ Cardiovasc Interv 5:605–614. https://doi.org/10.1161/CIRCINTERVENTIONS.112.971028
Hennessy RS, Go JL, Hennessy RR, Tefft BJ, Jana S, Stoyles NJ, Al-Hijji MA, Thaden JJ, Pislaru SV, Simari RD, Stulak JM, Young MD, Lerman A (2017) Recellularization of a novel off-the-shelf valve following xenogenic implantation into the right ventricular outflow tract. PLoS ONE 12:e0181614. https://doi.org/10.1371/journal.pone.0181614
Grebenik EA, Gafarova ER, Istranov LP, Istranova EV, Ma X, Xu J, Guo W, Atala A, Timashev PS (2020) Mammalian Pericardium-Based Bioprosthetic Materials in Xenotransplantation and Tissue Engineering. Biotechnol J 15:1–16. https://doi.org/10.1002/biot.201900334
L. Morticelli, D. Thomas, N. Roberts, E. Ingham, S. Korossis, Investigation of the suitability of decellularized porcine pericardium in mitral valve reconstruction., J. Heart Valve Dis. 22 (2013) 340–53. http://www.ncbi.nlm.nih.gov/pubmed/24151760.
Dalgliesh AJ, Parvizi M, Noble C, Griffiths LG (2019) Effect of cyclic deformation on xenogeneic heart valve biomaterials. PLoS ONE 14:e0214656. https://doi.org/10.1371/journal.pone.0214656
Whelan A, Williams E, Fitzpatrick E, Murphy BP, Gunning PS, O’Reilly D, Lally C (2021) Collagen fibre-mediated mechanical damage increases calcification of bovine pericardium for use in bioprosthetic heart valves. Acta Biomater. https://doi.org/10.1016/j.actbio.2021.04.046
Mirnajafi A, Zubiate B, Sacks MS (2010) Effects of cyclic flexural fatigue on porcine bioprosthetic heart valve heterograft biomaterials. J Biomed Mater Res - Part A 94:205–213. https://doi.org/10.1002/jbm.a.32659
Sacks MS, Schoen FJ (2002) Collagen fiber disruption occurs independent of calcification in clinically explanted bioprosthetic heart valves. J Biomed Mater Res 62:359–371. https://doi.org/10.1002/jbm.10293
Anssari-Benam A, Barber AH, Bucchi A (2016) Evaluation of bioprosthetic heart valve failure using a matrix-fibril shear stress transfer approach. J Mater Sci Mater Med 27:1–11. https://doi.org/10.1007/s10856-015-5657-2
C. Noble, M. Kamykowski, A. Lerman, M. Young (2020) Rate-Dependent and Relaxation Properties of Porcine Aortic Heart Valve Biomaterials, IEEE Open J. Eng. Med. Biol 1–1. https://doi.org/10.1109/OJEMB.2020.3002450.
Noble C, Choe J, Uthamaraj S, Deherrera M, Lerman A, Young M (2019) In Silico Performance of a Recellularized Tissue-Engineered Transcatheter Aortic Valve. J Biomech Eng 141:061004. https://doi.org/10.1115/1.4043209
Khorramirouz R, Go JL, Noble C, Morse D, Lerman A, Young MD (2019) In Vivo Response of Acellular Porcine Pericardial for Tissue Engineered Transcatheter Aortic Valves. Sci Rep 9:1094. https://doi.org/10.1038/s41598-018-37550-2
Choe JA, Jana S, Tefft BJ, Hennessy RS, Go J, Morse D, Lerman A, Young MD (2018) Biomaterial characterization of off-the-shelf decellularized porcine pericardial tissue for use in prosthetic valvular applications. J Tissue Eng Regen Med 12:1608–1620. https://doi.org/10.1002/term.2686
Noble C, Maxson EL, Lerman A, Young MD (2020) Mechanical and finite element evaluation of a bioprinted scaffold following recellularization in a rat subcutaneous model. J Mech Behav Biomed Mater 102:103519. https://doi.org/10.1016/j.jmbbm.2019.103519
Anssari-Benam A, Tseng YT, Bucchi A (2018) A transverse isotropic constitutive model for the aortic valve tissue incorporating rate-dependency and fibre dispersion: Application to biaxial deformation. J Mech Behav Biomed Mater 85:80–93. https://doi.org/10.1016/j.jmbbm.2018.05.035
Fehervary H, Smoljkić M, Vander Sloten J, Famaey N (2016) Planar biaxial testing of soft biological tissue using rakes: A critical analysis of protocol and fitting process. J. Mech. Behav. Biomed. Mater 61:135–151. https://doi.org/10.1016/j.jmbbm.2016.01.011
Kim H, Lu J, Sacks MS, Chandran KB (2008) Dynamic simulation of bioprosthetic heart valves using a stress resultant shell model. Ann Biomed Eng 36:262–275. https://doi.org/10.1007/s10439-007-9409-4
Noble C, Kamykowski M, Lerman A, Young M (2020) Rate-Dependent and Relaxation Properties of Porcine Aortic Heart Valve Biomaterials. IEEE Open J Eng Med Biol 1:1–1. https://doi.org/10.1109/OJEMB.2020.3002450
Sacks MS (2018) A Review on the Biomechanical Effects of Fatigue on the Porcine Bioprosthetic Heart Valve. J Long Term Eff Med Implants 27:181–197. https://doi.org/10.1615/jlongtermeffmedimplants.v27.i2-4.60
Martin C, Sun W (2013) Modeling of long-term fatigue damage of soft tissue with stress softening and permanent set effects. Biomech Model Mechanobiol 12:645–655. https://doi.org/10.1007/s10237-012-0431-6
Zhang W, Sacks MS (2017) Modeling the response of exogenously crosslinked tissue to cyclic loading: The effects of permanent set. J Mech Behav Biomed Mater 75:336–350. https://doi.org/10.1016/j.jmbbm.2017.07.013
Martin C, Sun W (2015) Fatigue damage of collagenous tissues: experiment, modeling and simulation studies. J Long Term Eff Med Implants 25:55–73. https://doi.org/10.1615/jlongtermeffmedimplants.2015011749
A. Rassoli, N. Fatouraee, R. Guidoin, Z. Zhang (2019) Comparison of tensile properties of xenopericardium from three animal species and finite element analysis for bioprosthetic heart valve tissue, Artif. Organs 1–10. https://doi.org/10.1111/aor.13552.
Aggarwal A, Pouch AM, Lai E, Lesicko J, Yushkevich PA, Gorman JH, Gorman RC, Sacks MS (2016) In-vivo heterogeneous functional and residual strains in human aortic valve leaflets. J Biomech 49:2481–2490. https://doi.org/10.1016/j.jbiomech.2016.04.038
Van Vlimmeren MAA, Driessen-Mol A, Oomens CWJ, Baaijens FPT (2012) Passive and active contributions to generated force and retraction in heart valve tissue engineering. Biomech Model Mechanobiol 11:1015–1027. https://doi.org/10.1007/s10237-011-0370-7
Sacks MS, Yoganathan AP (2007) Heart valve function: a biomechanical perspective. Philos Trans R Soc B Biol Sci 362:1369–1391. https://doi.org/10.1098/rstb.2007.2122
Acknowledgements
This work is supported by the HH Sheikh Hamed bin Zayed Al Nahyan Program in Biological Valve Engineering. Dr Christopher Noble is supported through the Mayo Clinic Career Development Award in Cardiovascular Diseases Research Honoring Dr. Earl H. Wood.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Noble, C., Morse, D., Lerman, A. et al. Evaluation of Pericardial Tissues from Assorted Species as a Tissue-Engineered Heart Valve Material. Med Biol Eng Comput 60, 393–406 (2022). https://doi.org/10.1007/s11517-021-02498-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-021-02498-5