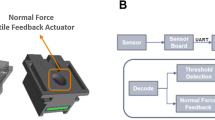
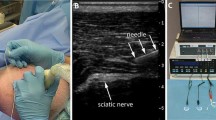
Abstract
Some tumours may not be detected by ultrasound during biopsy, but recent evidence has shown that different tissues can be discerned by electric impedance. This paper explores the application of vibrotactile feedback in an electrode embedded needle to help classify tissue during fine-needle aspiration biopsy from bioimpedance measurements. The process uses electric impedance spectroscopy from 10 Hz to 349 kHz to fit the double-dispersion Cole model through the Newton-Raphson algorithm. A Naive Bayes classifier is then used on the equivalent circuit parameters to estimate the tissue at the needle tip. The method is validated through a series of experiments and user trials. The results show that the vibrotactile feedback is able to help the operator in determining the tissue the needle is in, suggesting that this may prove to be a useful supplement to the standard procedure used today.

This paper explores the application of vibrotactile feedback for an electrode embedded needle to help classify tissue from electric impedance measurements. The data is fit to an equivalent circuit using Newton- Raphon’s method. A Naive Bayes classifier is trained and later used in an experiment to estimate the tissue at the needle tip and provide an assigned vibrotacticle feedback to the user.










Similar content being viewed by others
References
Åberg P, Birgersson U, Elsner P, Mohr P, Ollmar S (2011) Electrical impedance spectroscopy and the diagnostic accuracy for malignant melanoma. Exp Dermatol 20(8):648–652
Azimi P, Golnabi H (2009) Precise formulation of electrical capacitance for a cylindrical capacitive sensor. J Appl Sci 9(8):1556–1561
Bueschel P, Troeltzsch U, Kanoun O (2011) Use of stochastic methods for robust parameter extraction from impedance spectra. Electrochim Acta 56(23):8069–8077
Cole K (1941) Dispersion and absorption in dielectrics. J Chem Phys 9:341
De Luca CJ, Forrest WJ (1972) An electrode for recording single motor unit activity during strong muscle contractions. IEEE Trans Biomed Eng BME-19(5):367–372
Dodde R, Bull J, Shih A (2012) Bioimpedance of soft tissue under compression. Physiol Meas 33(6):1095
Ebeid A, Elshamy A (2018) Hypoechoic versus hypervascular lesion in the diagnosis of prostatic carcinoma. Afr J Urol 24(3):169–174
Freeborn TJ (2013) A survey of fractional-order circuit models for biology and biomedicine. IEEE J Emerg Sel Top Circ Syst 3(3):416–424
Freeborn TJ, Maundy B, Elwakil AS (2014) Extracting the parameters of the double-dispersion cole bioimpedance model from magnitude response measurements. Med Biol Eng Comput 52(9):749–758
Fricke H, Morse S (1925) The electric resistance and capacity of blood for frequencies between 800 and 41/2 million cycles. J Gen Physiol 9(2):153
Geddes L (1997) Historical evolution of circuit models for the electrode-electrolyte interface. Ann Biomed Eng 25(1):1
Grahame DC (1952) Mathematical theory of the faradaic admittance. J Electrochem Soc 99 (12):370–385
Graif T, Loeb S, Roehl KA, Gashti SN, Griffin C, Yu X, Catalona WJ (2007) Under diagnosis and over diagnosis of prostate cancer. J Urol 178(1):88–92
Green TC, Hogendijk M, Seiler K, DeSoto L, Ziring E (2004) Brachytherapy needle with impedance measurement apparatus and methods of use. US Patent 6,709,380
Grossi M, Riccò B (2017) Electrical impedance spectroscopy (EIS) for biological analysis and food characterization: a review. J Sens Sens Syst 6(2):303–325
Habibi M, Klemer DP, Raicu V (2010) Two-dimensional dielectric spectroscopy: Implementation and validation of a scanning open-ended coaxial probe. Rev Sci Instrum 81(7):075, 108
Halter RJ, Schned A, Heaney J, Hartov A, Paulsen KD (2009) Electrical properties of prostatic tissues: I. single frequency admittivity properties. J Urol 182(4):1600–1607
Halter RJ, Schned A, Heaney J, Hartov A, Schutz S, Paulsen KD (2008) Electrical impedance spectroscopy of benign and malignant prostatic tissues. The Journal of urology 179(4):1580–1586. https://doi.org/10.1016/j.juro.2007.11.043
Ihnatsenka B, Boezaart AP (2010) Ultrasound: basic understanding and learning the language. Int J Shoulder Surg 4(3):55
Ivorra A, Genescà M, Sola A, Palacios L, Villa R, Hotter G, Aguiló J (2005) Bioimpedance dispersion width as a parameter to monitor living tissues. Physiol Meas 26(2):S165
Jossinet J (1998) The impedivity of freshly excised human breast tissue. Physiol Meas 19(1):61
Kari J, Annala K, Annus P, Seppä VP, Kronström K (2015) A thin needle with bio-impedance measuring probe: tissue recognition performance assessed in in vivo animal study. Injeq Oy Ltd., Tech Rep
Kent B, Cusipag A, Rossa C (2019) Tissue discrimination through force-feedback from impedance spectroscopy in robot-assisted surgery. International Conference on Smart Multimedia, San Diego, Dec 2019. Accepted
Keshtkar A, Keshtkar A, Smallwood RH (2006) Electrical impedance spectroscopy and the diagnosis of bladder pathology. Physiol Meas 27(7):585
Korb KB, Nicholson AE (2010) Bayesian artificial intelligence. CRC press
Martinsen OG, Grimnes S Bioimpedance and bioelectricity basics. Academic press Third Edition
Min M, Lehti-Polojärvi M, Hyttinen J, Rist M, Land R, Annus P (2018) Bioimpedance spectro-tomography system using binary multifrequency excitation. Int J Bioelectromagn 209:76–79. https://doi.org/10.18154/RWTH-CONV-224930
Mishra V, Schned A, Hartov A, Heaney J, Seigne J, Halter R (2013) Electrical property sensing biopsy needle for prostate cancer detection. Prostate 73(15):1603–1613
Nahir TM (2005) Impedance spectroscopy: Theory, experiment, and applications, edited by evgenij barsoukov (texas instruments inc.) and j. ross macdonald (university of north carolina, chapel hill). john wiley & sons, inc.: Hoboken, nj. 2005. xvii+ 596 pp isbn 0471-64749-7
Nejadgholi I, Caytak H, Bolic M, Batkin I, Shirmohammadi S (2015) Preprocessing and parameterizing bioimpedance spectroscopy measurements by singular value decomposition. Physiol Meas 36(5):983
Norberg M, Egevad L, Holmberg L, Sparen P, Norlen B, Busch C (1997) The sextant protocol for ultrasound-guided core biopsies of the prostate underestimates the presence of cancer. Urology 50 (4):562–566
Okamura AM (2004) Methods for haptic feedback in teleoperated robot-assisted surgery. Ind Robot: An Int J 31(6):499–508
Ortega JM, Rheinboldt WC (1970) Iterative solution of nonlinear equations in several variables, vol. 30. Siam. Edition from 2000 republication of 1970 publication
Platt G (2020) How do i convert a continuous-time model to a discrete-time model? Coursera Inc. Accessed: 2020-03-06. https://www.coursera.org/lecture/equivalent-circuit-cell-modelsimulation/2-1-5-how-do-i-convert-a-continuous-time-model-to-a-discretetime-model-ZTWU4
Westebring-van der Putten EP, Goossens RH, Jakimowicz JJ, Dankelman J (2008) Haptics in minimally invasive surgery–a review. Minim Invasive Ther Allied Technol 17(1):3–16
Randles JEB (1947) Kinetics of rapid electrode reactions. Discuss Faraday Soc 1:11–19
Raschka S (2014) Naive bayes and text classification i-introduction and theory. arXiv:1410.5329
Rigaud B, Hamzaoui L, Frikha M, Chauveau N, Morucci JP (1995) In vitro tissue characterization and modelling using electrical impedance measurements in the 100 hz-10 mhz frequency range. Physiol Meas 16(3A):A15
Theodoridis S (2015) Machine learning: a Bayesian and optimization perspective. Academic Press
Trebbels D, Fellhauer F, Jugl M, Haimerl G, Min M, Zengerle R (2011) Online tissue discrimination for transcutaneous needle guidance applications using broadband impedance spectroscopy. IEEE Trans Biomed Eng 59(2):494–503
Warburg E (1899) Ueber das verhalten sogenannter unpolarisirbarer elektroden gegen wechselstrom. Ann Phys 303(3):493–499
Wasterlain S, Candusso D, Harel F, François X., Hissel D (2010) Diagnosis of a fuel cell stack using electrochemical impedance spectroscopy and bayesian networks. In: 2010 IEEE vehicle power and propulsion conference. IEEE, pp 1–6
Wu Q, Bell D, McGinnity M, Guo G (2005) Decision making based on hybrid of multi-knowledge and naïve bayes classifier. In: Foundations of data mining and knowledge discovery. Springer, pp 171–184
Yorkey TJ, Webster JG, Tompkins WJ (1987) Comparing reconstruction algorithms for electrical impedance tomography. IEEE Trans Biomed Eng BME-34(11):843–852
Yun J, Hong YT, Hong KH, Lee JH (2018) Ex vivo identification of thyroid cancer tissue using electrical impedance spectroscopy on a needle. Sens Actuators B 261:537–544
Funding
We acknowledge the support of the Natural Sciences and Engineering Research Council of Canada (NSERC), the Canadian Institutes of Health Research (CIHR), and the Social Sciences and Humanities Research Council of Canada (SSHRC) [funding reference number NFRFE-2018-01986]. Cette recherche a été financée par le Conseil de recherches en sciences naturelles et en génie du Canada (CRSNG), par les Instituts de recherche en santé du Canada (IRSC), et par le Conseil de recherches en sciences humaines du Canada (CRSH), [numéro de référence NFRFE-2018-01986]
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kent, B., Rossa, C. Development of a tissue discrimination electrode embedded surgical needle using vibro-tactile feedback derived from electric impedance spectroscopy. Med Biol Eng Comput 60, 19–31 (2022). https://doi.org/10.1007/s11517-021-02454-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-021-02454-3