Abstract
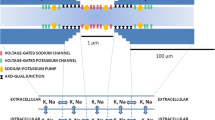
Biophysical computational models of axons provide an important tool for quantifying the effects of injury and disease on signal conduction characteristics. Several studies have used generic models to study the average behavior of healthy and injured axons; however, few studies have included the effects of normal structural variation on the simulated axon’s response to injury. The effects of variations in physiological characteristics on axonal function were mapped by altering the structure of the nodal, paranodal, and juxtaparanodal regions across reported values in three different caliber axons (1, 2, and 5.7 μm). Myelin detachment and retraction were simulated to quantify the effects of each injury mechanism on signal conduction. Conduction velocity was most affected by axonal fiber diameter (89%), while membrane potential amplitude was most affected by nodal length (86%) in healthy axons. Postinjury axonal functionality was most affected by myelin detachment in the paranodal and juxtaparanodal regions when retraction and detachment were modeled simultaneously. The efficacy of simulated potassium channel blockers on restoring membrane potential and velocity varied with axonal caliber and injury type. The structural characteristics of axons affect their functional response to myelin retraction and detachment and their subsequent response to potassium channel blocker treatment.










Similar content being viewed by others
References
Alluin O, Delivet-Mongrain H, Gauthier M-K, Fehlings MG, Rossignol S, Karimi-Abdolrezaee S (2014) Examination of the combined effects of chondroitinase ABC, growth factors and locomotor training following compressive spinal cord injury on neuroanatomical plasticity and kinematics. PLoS One 9:e111072. doi:10.1371/journal.pone.0111072
Anthes DL, Theriault E, Tator CH (1995) Characterization of axonal ultrastructural pathology following experimental spinal cord compression injury. Brain Res 702:1–16. doi:10.1016/0006-8993(95)01028-6
Arancibia-Carcamo IL, Attwell D (2014) The node of Ranvier in CNS pathology. Acta Neuropathol 128:161–175. doi:10.1007/s00401-014-1305-z
Arlow RL, Foutz TJ, McIntyre CC (2013) Theoretical principles underlying optical stimulation of myelinated axons expressing channelrhodopsin-2. Neuroscience 248:541–551. doi:10.1016/j.neuroscience.2013.06.031
Babbs CF, Shi R (2013) Subtle paranodal injury slows impulse conduction in a mathematical model of myelinated axons. PLoS One 8:e67767
Berthold CH, Nilsson I, Rydmark M (1983) Axon diameter and myelin sheath thickness in nerve fibres of the ventral spinal root of the seventh lumbar nerve of the adult and developing cat. J Anat 136:483
Berthold CH, Rydmark M (1983) Anatomy of the paranode-node-paranode region in the cat. Cell Mol Life Sci 39:964–976
Bhadra N, Lahowetz EA, Foldes ST, Kilgore KL (2007) Simulation of high-frequency sinusoidal electrical block of mammalian myelinated axons. J Comput Neurosci 22:313–326. doi:10.1007/s10827-006-0015-5
Blight AR (1983) Cellular morphology of chronic spinal cord injury in the cat: analysis of myelinated axons by line-sampling. Neuroscience 10:521–543. doi:10.1016/0306-4522(83)90150-1
Blight AR (1989) Effect of 4-aminopyridine on axonal conduction-block in chronic spinal cord injury. Brain Res Bull 22:47–52. doi:10.1016/0361-9230(89)90126-3
Blight AR (1991) Morphometric analysis of a model of spinal cord injury in guinea pigs, with behavioral evidence of delayed secondary pathology. J Neurol Sci 103:156–171. doi:10.1016/0022-510X(91)90159-5
Bostock H, Sears TA, Sherratt RM (1983) The spatial distribution of excitability and membrane current in normal and demyelinated mammalian nerve fibres. J Physiol 341:41–58. doi:10.1113/jphysiol.1981.sp013666
Boucher P-A, Joós B, Morris CE (2012) Coupled left-shift of Nav channels: modeling the Na -loading and dysfunctional excitability of damaged axons. J Comput Neurosci 33:301–319
Boyd IA, Kalu KU (1979) Scaling factor relating conduction velocity and diameter for myelinated afferent nerve fibres in the cat hind limb. J Physiol 289:277–297
Van der Bruggen MA, Huisman HB, Beckerman H, Bertelsmann FW, Polman CH, Lankhorst GJ (2001) Randomized trial of 4-aminopyridine in patients with chronic incomplete spinal cord injury. J Neurol 248:665–671. doi:10.1007/s004150170111
Capogrosso M, Wenger N, Raspopovic S, Musienko P, Beauparlant J, Bassi Luciani L, Courtine G, Micera S (2013) A computational model for epidural electrical stimulation of spinal sensorimotor circuits. J Neurosci 33:19326–19340. doi:10.1523/JNEUROSCI.1688-13.2013
Carnevale NT, Hines ML (2006) The NEURON book. Neuron. doi:10.1017/CBO9780511541612
Castelfranco AM, Hartline DK (2015) The evolution of vertebrate and invertebrate myelin: a theoretical computational study. J Comput Neurosci 38:521–538. doi:10.1007/s10827-015-0552-x
Danner SM, Hofstoetter US, Ladenbauer J, Rattay F, Minassian K (2011) Can the human lumbar posterior columns be stimulated by transcutaneous spinal cord stimulation? A modeling study. Artif Organs 35:257–262
DeForge D, Nymark J, Lemaire E, Gardner S, Hunt M, Martel L, Curran D, Barbeau H (2004) Effect of 4-aminopyridine on gait in ambulatory spinal cord injuries: a double-blind, placebo-controlled, crossover trial. Spinal Cord Off J Int Med Soc Paraplegia. doi:10.1038/sj.sc.3101653
Devaux J, Gow A (2008) Tight junctions potentiate the insulative properties of small CNS myelinated axons. J Cell Biol 183:909–921. doi:10.1083/jcb.200808034
Van Dijk KJ, Verhagen R, Chaturvedi A, McIntyre CC, Bour LJ, Heida C, Veltink PH (2015) A novel lead design enables selective deep brain stimulation of neural populations in the subthalamic region. J Neural Eng 12:46003
Domingo A, Al-Yahya AA, Asiri Y, Eng JJ, Lam T, Spinal Cord Injury Rehabilitation Evidence Research Team (2012) A systematic review of the effects of pharmacological agents on walking function in people with spinal cord injury. J Neurotrauma 29:865–879. doi:10.1089/neu.2011.2052
Espósito MS, Piatti VC, Laplagne DA, Morgenstern NA, Ferrari CC, Pitossi FJ, Schinder AF (2005) Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J Neurosci 25:10074–10086
Fiore MS (1981) Atlas of human histology. Lea & Febiger, Philadelphia
Fu Y, Sun W, Shi Y, Shi R, Cheng JX (2009) Glutamate excitotoxicity inflicts paranodal myelin splitting and retraction. PLoS One. doi:10.1371/journal.pone.0006705
Gasser H, Grundfest H (1939) Axon diameters in relation to the spike dimensions and the conduction velocity in mammalian A fibers. Am J Phys 127:393–414
Geddes DM, LaPlaca MC, Cargill RS (2003) Susceptibility of hippocampal neurons to mechanically induced injury. Exp Neurol 184:420–427. doi:10.1016/S0014-4886(03)00254-1
Goldman L, Albus JS (1968) Computation of impulse conduction in myelinated fibers; theoretical basis of the velocity-diameter relation. Biophys J 8:596–607. doi:10.1016/S0006-3495(68)86510-5
Hartline DK, Colman DR (2007) Rapid conduction and the evolution of giant axons and myelinated fibers. Curr Biol 17:R29–R35. doi:10.1016/j.cub.2006.11.042
Hernández-Labrado GR, Polo JL, López-Dolado E, Collazos-Castro JE (2011) Spinal cord direct current stimulation: finite element analysis of the electric field and current density. Med Biol Eng Comput 49:417–429. doi:10.1007/s11517-011-0756-9
Hines ML, Morse T, Migliore M, Carnevale NT, Shepherd GM (2004) ModelDB: a database to support computational neuroscience. J Comput Neurosci 17:7–11
Hoffer JA, Loeb GE, Marks WB, O’Donovan MJ, Pratt CA, Sugano N (1987) Cat hindlimb motoneurons during locomotion. I. Destination, axonal conduction velocity, and recruitment threshold. J Neurophysiol 57:510–529
Holt AB, Netoff TI (2013) Computational modeling of epilepsy for an experimental neurologist. Exp Neurol 244:75–86. doi:10.1016/j.expneurol.2012.05.003
Horresh I, Poliak S, Grant S, Bredt D, Rasband MN, Peles E (2008) Multiple molecular interactions determine the clustering of Caspr2 and Kv1 channels in myelinated axons. J Neurosci 28:14213–14222. doi:10.1523/JNEUROSCI.3398-08.2008
Iwata A, Stys PK, Wolf JA, Chen XH, Taylor AG, Meaney DF, Smith DH (2004) Traumatic axonal injury induces proteolytic cleavage of the voltage-gated sodium channels modulated by tetrodotoxin and protease inhibitors. J Neurosci 24:4605–4613. doi:10.1523/JNEUROSCI.0515-03.2004
Jensen JM, Shi R (2003) Effects of 4-aminopyridine on stretched mammalian spinal cord: the role of potassium channels in axonal conduction. J Neurophysiol 90:2334–2340. doi:10.1152/jn.00868.2002
Jérusalem A, García-Grajales JA, Merchán-Pérez A, Peña JM (2013) A computational model coupling mechanics and electrophysiology in spinal cord injury. Biomech Model Mechanobiol 13:1–14. doi:10.1007/s10237-013-0543-7
Johnson MD, McIntyre CC (2008) Quantifying the neural elements activated and inhibited by globus pallidus deep brain stimulation. J Neurophysiol 100:2549–2563. doi:10.1152/jn.90372.2008
Kerr JND, Wickens JR (2001) Dopamine D-1/D-5 receptor activation is required for long-term potentiation in the rat neostriatum in vitro. J Neurophysiol 85:117–124
Khakshour S, Beischlag TV, Sparrey C, Park EJ (2015) Probing mechanical properties of Jurkat cells under the effect of ART using oscillating optical tweezers. PLoS One. doi:10.1371/journal.pone.0126548
Kolaric KV, Thomson G, Edgar JM, Brown AM (2013) Focal axonal swellings and associated ultrastructural changes attenuate conduction velocity in central nervous system axons: a computer modeling study. Physiol Rep 1:e00059. doi:10.1002/phy2.59
Lacour SP, Tsay C, Wagner S, Zhe Y, Morrison B (2005) Stretchable micro-electrode arrays for dynamic neuronal recording of in vitro mechanically injured brain. In: Proc. IEEE Sensors. pp 617–620
Lempka SF (2010) The electrode-tissue interface during recording and stimulation in the central nervous system. Diss. Case Western Reserve University
Leung G, Sun W, Brookes S, Smith D, Shi R (2011) Potassium channel blocker, 4-aminopyridine-3-methanol, restores axonal conduction in spinal cord of an animal model of multiple sclerosis. Exp Neurol 227:232–235
Lovas G, Szilagyi N, Majtenyi K, Palkovits M, Komoly S (2000) Axonal changes in chronic demyelinated cervical spinal cord plaques. Brain 123:308–317. doi:10.1093/brain/123.2.308
Lujan JL, Chaturvedi A, Choi KS, Holtzheimer PE, Gross RE, Mayberg HS, McIntyre CC (2013) Tractography-activation models applied to subcallosal cingulate deep brain stimulation. Brain Stimul 6:737–739. doi:10.1016/j.brs.2013.03.008
Makeig S, Kothe C, Mullen T, Bigdely-Shamlo N, Zhang Z, Kreutz-Delgado K (2012) Evolving signal processing for brain–computer interfaces. Proc IEEE 100:1567–1584
Mcintyre CC, Grill WM (2002) Extracellular stimulation of central neurons: influence of stimulus waveform and frequency on neuronal output. J Neurophysiol 88:1592–1604
McIntyre CC, Grill WM, Sherman DL, Thakor NV (2004) Cellular effects of deep brain stimulation: model-based analysis of activation and inhibition. J Neurophysiol 91:1457–1469. doi:10.1152/jn.00989.2003
McIntyre CC, Richardson AG, Grill WM (2002) Modeling the excitability of mammalian nerve fibers: influence of afterpotentials on the recovery cycle. J Neurophysiol 87:995–1006. doi:10.1152/jn.00353.2001
Moffitt MA, McIntyre CC, Grill WM (2004) Prediction of myelinated nerve fiber stimulation thresholds: limitations of linear models. IEEE Trans Biomed Eng 51:229–236. doi:10.1109/TBME.2003.820382
More HL, Hutchinson JR, Collins DF, Weber DJ, Aung SKH, Donelan JM (2010) Scaling of sensorimotor control in terrestrial mammals. Proc R Soc B Biol Sci 277:3563–3568. doi:10.1098/rspb.2010.0898
Nashmi R, Fehlings MG (2001) Mechanisms of axonal dysfunction after spinal cord injury: with an emphasis on the role of voltage-gated potassium channels. Brain Res Rev 38:165–191. doi:10.1016/S0165-0173(01)00134-5
Nilsson I, Berthold CH (1988) Axon classes and internodal growth in the ventral spinal root L7 of adult and developing cats. J Anat 156:71–96
Nowak LG, Bullier J (1998) Axons, but not cell bodies, are activated by electrical stimulation in cortical gray matter. Exp Brain Res 118:489–500. doi:10.1007/s002210050305
Ong HH, Wehrli FW (2010) Quantifying axon diameter and intra-cellular volume fraction in excised mouse spinal cord with q-space imaging. NeuroImage 51:1360–1366. doi:10.1016/j.neuroimage.2010.03.063
Ouyang H, Sun W, Fu Y, Li J, Cheng J-X, Nauman E, Shi R (2010) Compression induces acute demyelination and potassium channel exposure in spinal cord. J Neurotrauma 27:1109–1120. doi:10.1089/neu.2010.1271
Pelot NA, Behrend CE, Grill WM (2015) Modeling the response of small myelinated and unmyelinated axons to kilohertz frequency signals. In: 2015 7th International IEEE/EMBS Conference on Neural Engineering (NER), Montpellier, pp 406–409. doi:10.1109/NER.2015.7146645
Perge JA, Niven JE, Mugnaini E, Balasubramanian V, Sterling P (2012) Why do axons differ in caliber? J Neurosci 32:626–638. doi:10.1523/JNEUROSCI.4254-11.2012
Powers BE, Lasiene J, Plemel JR, Shupe L, Perlmutter SI, Tetzlaff W, Horner PJ (2012) Axonal thinning and extensive remyelination without chronic demyelination in spinal injured rats. J Neurosci 32:5120–5125. doi:10.1523/JNEUROSCI.0002-12.2012
Ragnarsson KT (2008) Functional electrical stimulation after spinal cord injury: current use, therapeutic effects and future directions. Spinal Cord 46:255–274. doi:10.1038/sj.sc.3102091
Ranck JB (1975) Which elements are excited in electrical stimulation of mammalian central nervous system: a review. Brain Res 98:417–440. doi:10.1016/0006-8993(75)90364-9
Reeves TM, Greer JE, Vanderveer AS, Phillips LL (2010) Proteolysis of submembrane cytoskeletal proteins ankyrin-G and alpha-II-spectrin following diffuse brain injury: a role in white matter vulnerability at nodes of Ranvier. Brain Pathol 20:1055–1068. doi:10.1111/j.1750-3639.2010.00412.x
Reeves TM, Phillips LL, Povlishock JT (2005) Myelinated and unmyelinated axons of the corpus callosum differ in vulnerability and functional recovery following traumatic brain injury. Exp Neurol 196:126–137
Rosenberg LJ, Wrathall JR (1997) Quantitative analysis of acute axonal pathology in experimental spinal cord contusion. J Neurotrauma 14:823–838. doi:10.1089/neu.1997.14.823
Russell CM, Choo AM, Tetzlaff W, Chung T-E, Oxland TR (2012) Maximum principal strain correlates with spinal cord tissue damage in contusion and dislocation injuries in the rat cervical spine. J Neurotrauma 29:1574–1585. doi:10.1089/neu.2011.2225
Rydmark M (1981) Nodal axon diameter correlates linearly with internodal axon diameter in spinal roots of the cat. Neurosci Lett 24:247–250
Rydmark M, Berthold CH (1983) Electron microscopic serial section analysis of nodes of Ranvier in lumbar spinal roots of the cat: a morphometric study of nodal compartments in fibres of different sizes. J Neurocytol 12:537–565
Schwarz JR, Reid G, Bostock H (1995) Action potentials and membrane currents in the human node of Ranvier. Eur J Phys 430:283–292. doi:10.1007/BF00374660
Shi R, Blight AR (1996) Compression injury of mammalian spinal cord in vitro and the dynamics of action potential conduction failure. J Neurophysiol 76:1572–1580
Shi R, Pryor JD (2002) Pathological changes of isolated spinal cord axons in response to mechanical stretch. Neuroscience 110:765–777
Shi R, Whitebone J (2006) Conduction deficits and membrane disruption of spinal cord axons as a function of magnitude and rate of strain. J Neurophysiol 95:3384–3390. doi:10.1152/jn.00350.2005
Smith KJ, Felts PA, John GR (2000) Effects of 4-aminopyridine on demyelinated axons, synapses and muscle tension. Brain 123:171–184
Sterratt D, Graham B, Gillies A, Willshaw D (2011) Principles of computational modelling in neuroscience. Cambridge University Press doi:10.1017/CBO9780511975899
Stirling DP, Stys PK (2010) Mechanisms of axonal injury: internodal nanocomplexes and calcium deregulation. Trends Mol Med 16:160–170. doi:10.1016/j.molmed.2010.02.002
Streijger F, Lee JHT, Duncan GJ, Ng MTL, Assinck P, Bhatnagar T, Plunet WT, Tetzlaff W, Kwon BK (2014) Combinatorial treatment of acute spinal cord injury with ghrelin, ibuprofen, C16, and ketogenic diet does not result in improved histologic or functional outcome. J Neurosci Res 92:870–883. doi:10.1002/jnr.23372
Sun W, Fu Y, Shi Y, Cheng J-X, Cao P, Shi R (2012) Paranodal myelin damage after acute stretch in guinea pig spinal cord. J Neurotrauma 29:611–619
Sun W, Smith D, Fu Y, Cheng J-X, Bryn S, Borgens R, Shi R (2010) Novel potassium channel blocker, 4-AP-3-MeOH, inhibits fast potassium channels and restores axonal conduction in injured guinea pig spinal cord white matter. J Neurophysiol 103:469–478. doi:10.1152/jn.00154.2009
Susuki K (2013) Node of Ranvier disruption as a cause of neurological diseases. ASN Neuro 5:209–219. doi:10.1042/AN20130025
Tang-Schomer MD, Johnson VE, Baas PW, Stewart W, Smith DH (2012) Partial interruption of axonal transport due to microtubule breakage accounts for the formation of periodic varicosities after traumatic axonal injury. Exp Neurol 233:364–372. doi:10.1016/j.expneurol.2011.10.030
Tripathy SJ, Savitskaya J, Burton SD, Urban NN, Gerkin RC (2014) NeuroElectro: a window to the world’s neuron electrophysiology data. Front Neuroinform 8:40. doi:10.3389/fninf.2014.00040
Tseng K, Li H, Clark A, Sundem L, Zuscik M, Noble M, Elfar J (2016) 4-Aminopyridine promotes functional recovery and remyelination in acute peripheral nerve injury. EMBO Mol Med 8:1409–LP-1420
Varma AK, Das A, Wallace G, Barry J, Vertegel AA, Ray SK, Banik NL (2013) Spinal cord injury: a review of current therapy, future treatments, and basic science frontiers. Neurochem Res 38:895–905. doi:10.1007/s11064-013-0991-6
Vodovnik L, Stefanovska A, Bajd T (1987) Effects of stimulation parameters on modification of spinal spasticity. Med Biol Eng Comput 25:439–442. doi:10.1007/BF02443365
Volman V, Ng LJ (2013) Computer modeling of mild axonal injury: implications for axonal signal transmission. Neural Comput 25:2646–2681
Volman V, Ng LJ (2014) Primary paranode demyelination modulates slowly developing axonal depolarization in a model of axonal injury. J Comput Neurosci:439–457. doi:10.1007/s10827-014-0515-7
Wang H, Fu Y, Zickmund P, Shi R, Cheng J-X (2005) Coherent anti-stokes Raman scattering imaging of axonal myelin in live spinal tissues. Biophys J 89:581–591. doi:10.1529/biophysj.105.061911
Waxman SG (2006) Axonal conduction and injury in multiple sclerosis: the role of sodium channels. Nat Rev Neurosci 7:932–941. doi:10.1038/nrn2023
Waxman SG, Kocsis JD, Stys PK (1995) The axon: structure, function, and pathophysiology. Oxford University Press
Wolfe DL, Hayes KC, Hsieh JT, Potter PJ (2001) Effects of 4-aminopyridine on motor evoked potentials in patients with spinal cord injury: a double-blinded, placebo-controlled crossover trial. J Neurotrauma. doi:10.1089/089771501316919120
Zhu F, Kuhlmann MK, Kaysen GA, Sarkar S, Kaitwatcharachai C, Khilnani R, Stevens L, Leonard EF, Wang J, Heymsfield S, Levin NW (2006) Segment-specific resistivity improves body fluid volume estimates from bioimpedance spectroscopy in hemodialysis patients. J Appl Physiol 100:717–724. doi:10.1152/japplphysiol.00669.2005
Acknowledgements
This work was supported by the Natural Sciences and Engineering Council of Canada (NSERC) and Simon Fraser University. We would like to thank Dr. Farzaneh Davoodi for her comments on the article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
About this article
Cite this article
Daneshi Kohan, E., Lashkari, B.S. & Sparrey, C.J. The effects of paranodal myelin damage on action potential depend on axonal structure. Med Biol Eng Comput 56, 395–411 (2018). https://doi.org/10.1007/s11517-017-1691-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-017-1691-1