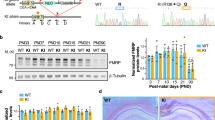
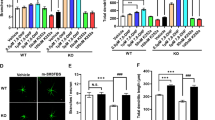
Abstract
Fragile X syndrome (FXS) is the most common monogenic cause of intellectual disability and a cause for autism. FXS females report milder phenotypes and a lower rate of cognitive problems compared to males. This is most likely because most females are heterozygous, while males are hemizygous for the disease. Thus, most preclinical studies have been completed in males. As there is major interest in testing experimental drugs for FXS, it is imperative to determine whether females in animal models used for research, present behavioral alterations that might translate to humans in order to confirm that experimental drugs have an effect on both genders. In our study we describe behavioral phenotypes in homozygous FXS female mice developed on the FVB.129 background. We focused on detection of hippocampal-mediated cognitive abilities and other behaviors described for FXS. Our research shows that, while female FVB.129-Fmr1 knockout mice present normal learning, they have impaired memory, as well as susceptibility to audiogenic seizures. In agreement with previous reports in rodents and humans, significant levels of the small GTPase Rac1 were found in FXS female mice. Because Rac1 is involved in neuronal development, plasticity and behavior, we additionally aimed to pharmacologically inhibit Rac1 and determine whether observed phenotypes are rescued. Treatment of female FVB.129-Fmr1 knockout with a Rac1 inhibitor abolished behavioral deficits, bringing phenotypes to control levels. Our results suggest that female FVB.129-Fmr1 knockout mice display behavioral impairments that resemble FXS in humans. Moreover, those behavioral shortfalls might be associated with alteration of plasticity involving excessive Rac1 function, since pharmacological reduction of Rac1 normalizes previously altered phenotypes to control levels.
Similar content being viewed by others
References
Bailey D B, Hatton D D, Skinner M (1998). Early developmental trajectories of males with fragile X syndrome. Am J Ment Retard, 103(1): 29–39
Baker K B, Wray S P, Ritter R, Mason S, Lanthorn T H, Savelieva K V (2010). Male and female Fmr1 knockout mice on C57 albino background exhibit spatial learning and memory impairments. Genes Brain Behav, 9(6): 562–574
Bi R, Broutman G, Foy M R, Thompson R F, Baudry M (2000). The tyrosine kinase and mitogen-activated protein kinase pathways mediate multiple effects of estrogen in hippocampus. Proc Natl Acad Sci USA, 97(7): 3602–3607
Bi R, Foy MR, Vouimba RM, Thompson R F, BaudryM(2001). Cyclic changes in estradiol regulate synaptic plasticity through the MAP kinase pathway. Proc Natl Acad Sci USA, 98(23): 13391–13395
Bongmba O Y, Martinez L A, ElhardtME, Butler K, Tejada-SimonMV (2011). Modulation of dendritic spines and synaptic function by Rac1: a possible link to Fragile X syndrome pathology. Brain Res, 1399: 79–95
Chen L, Toth M (2001). Fragile X mice develop sensory hyperreactivity to auditory stimuli. Neuroscience, 103(4): 1043–1050
Crawley J N (2000). What’s wrong with my mouse? Behavioral phenotyping of transgenic and knockout mice. Wiley-Liss, John Wiley and Sons, Inc.
Ding Q, Sethna F, Wang H (2014). Behavioral analysis of male and female Fmr1 knockout mice on C57BL/6 background. Behav Brain Res, 271: 72–78
Fan L, Zhao Z, Orr P T, Chambers C H, Lewis M C, Frick K M (2010). Estradiol-induced object memory consolidation in middle-aged female mice requires dorsal hippocampal extracellular signalregulated kinase and phosphatidylinositol 3-kinase activation. J Neurosci, 30(12): 4390–4400
Fatemi S H, Folsom T D, Kneeland R E, Yousefi M K, Liesch S B, Thuras P D (2013). Impairment of fragile X mental retardation protein-metabotropic glutamate receptor 5 signaling and its downstream cognates ras-related C3 botulinum toxin substrate 1, amyloid beta A4 precursor protein, striatal-enriched protein tyrosine phosphatase, and homer 1, in autism: a postmortem study in cerebellar vermis and superior frontal cortex. Mol Autism, 4(1): 21–26
Fernandez S M, Lewis M C, Pechenino A S, Harburger L L, Orr P T, Gresack J E, Schafe G E, Frick K M (2008). Estradiol-induced enhancement of object memory consolidation involves hippocampal ERK activation and membrane-bound estrogen receptors. J Neurosci, 28: 8660–8667
Fortress A M, Fan L, Orr P T, Zhao Z, Frick K M (2013). Estradiolinduced object recognition memory consolidation is dependent on activation of mTOR signaling in the dorsal hippocampus. Learn Mem, 20(3): 147–155
Frankland PW, Wang Y, Rosner B, Shimizu T, Balleine BW, Dykens E M, Ornitz E M, Silva A J (2004). Sensorimotor gating abnormalities in young males with fragile X syndrome and Fmr1-knockout mice. Mol Psychiatry, 9(4): 417–425
Garcia-Segura L M, Wozniak A, Azcoitia I, Rodriguez J R, Hutchison R E, Hutchison J B (1999). Aromatase expression by astrocytes after brain injury: implications for local estrogen formation in brain repair. Neuroscience, 89(2): 567–578
Goebel-Goody S M, Wilson-Wallis E D, Royston S, Tagliatela S M, Naegele J R, Lombroso P J (2012). Genetic manipulation of STEP reverses behavioral abnormalities in a fragile X syndrome mouse model. Genes Brain Behav, 11(5): 586–600
Gould E, Woolley C S, Frankfurt M, McEwen B S (1990). Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci, 10(4): 1286–1291
Hasegawa Y, Hojo Y, Kojima H, Ikeda M, Hotta K, Sato R, Ooishi Y, Yoshiya M, Chung B C, Yamazaki T, Kawato S (2015). Estradiol rapidly modulates synaptic plasticity of hippocampal neurons: Involvement of kinase networks. Brain Res, 1621: 147–161
Hojo Y, Hattori T A, Enami T, Furukawa A, Suzuki K, Ishii H T, Mukai H, Morrison J H, Janssen W G, Kominami S, Harada N, Kimoto T, Kawato S (2004). Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017alpha and P450 aromatase localized in neurons. Proc Natl Acad Sci USA, 101(3): 865–870
Hojo Y, Higo S, Ishii H, Ooishi Y, Mukai H, Murakami G, Kominami T, Kimoto T, Honma S, Poirier D, Kawato S (2009). Comparison between hippocampus-synthesized and circulation-derived sex steroids in the hippocampus. Endocrinology, 150(11): 5106–5112
Kato A, Hojo Y, Higo S, Komatsuzaki Y, Murakami G, Yoshino H, Uebayashi M, Kawato S (2013). Female hippocampal estrogens have a significant correlation with cyclic fluctuation of hippocampal spines. Front Neural Circuits, 7: 149
Kramár E A, Chen L Y, Brandon N J, Rex C S, Liu F, Gall CM, Lynch G (2009). Cytoskeletal changes underlie estrogen’s acute effects on synaptic transmission and plasticity. J Neurosci, 29(41): 12982–12993
Kretz O, Fester L, Wehrenberg U, Zhou L, Brauckmann S, Zhao S, Prange-Kiel J, Naumann T, Jarry H, Frotscher M, Rune G M (2004). Hippocampal synapses depend on hippocampal estrogen synthesis. J Neurosci, 24(26): 5913–5921
Le Doux J (1996). Emotional networks and motor control: a fearful view. Prog Brain Res, 107: 437–446
Lesniak-Karpiak K, Mazzocco M M, Ross J L (2003). Behavioral assessment of social anxiety in females with Turner or fragile X syndrome. J Autism Dev Disord, 33(1): 55–67
Levay M, Krobert K A, Wittig K, Voigt N, Bermudez M, Wolber G, Dobrev D, Levy F O, Wieland T (2013). NSC23766, a widely used inhibitor of Rac1 activation, additionally acts as a competitive antagonist at muscarinic acetylcholine receptors. J Pharmacol Exp Ther, 347(1): 69–79
Lewis M C, Kerr K M, Orr P T, Frick K M (2008). Estradiol-induced enhancement of object memory consolidation involves NMDA receptors and protein kinase A in the dorsal hippocampus of female C57BL/6 mice. Behav Neurosci, 122(3): 716–721
Martinez L A, Tejada-Simon M V (2011). Pharmacological inactivation of the small GTPase Rac1 impairs long-term plasticity in the mouse hippocampus. Neuropharmacology, 61(1-2): 305–312
Mazzocco M M, Baumgardner T, Freund L S, Reiss A L (1998). Social functioning among girls with fragile X or Turner syndrome and their sisters. J Autism Dev Disord, 28(6): 509–517
McCauley E, Feuillan P, Kushner H, Ross J L (2001). Psychosocial development in adolescents with Turner syndrome. J Dev Behav Pediatr, 22(6): 360–365
Michelson H B, Lothman E W (1989). An in vivo electrophysiological study of the ontogeny of excitatory and inhibitory processes in the rat hippocampus. Brain Res Dev Brain Res, 47(1): 113–122
Musumeci S A, Bosco P, Calabrese G, Bakker C, De Sarro G B, Elia M, Ferri R, Oostra B A (2000). Audiogenic seizures susceptibility in transgenic mice with fragile X syndrome. Epilepsia, 41(1): 19–23
Nielsen D M, DerberWJ, McClellan D A, Crnic L S (2002). Alterations in the auditory startle response in Fmr1 targeted mutant mouse models of fragile X syndrome. Brain Res, 927(1): 8–17
Paylor R, Crawley J N (1997). Inbred strain differences in prepulse inhibition of the mouse startle response. Psychopharmacology (Berl), 132(2): 169–180
Pellow S, Chopin P, File S E, BrileyM(1985). Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods, 14(3): 149–167
Pietropaolo S, Guilleminot A, Martin B, D’Amato F R, Crusio W E (2011). Genetic-background modulation of core and variable autisticlike symptoms in Fmr1 knock-out mice. PLoS ONE, 6(2): e17073
Prange-Kiel J, Fester L, Zhou L, Lauke H, Carrétero J, Rune G M (2006). Inhibition of hippocampal estrogen synthesis causes regionspecific downregulation of synaptic protein expression in hippocampal neurons. Hippocampus, 16(5): 464–471
Rousseau F, Heitz D, Tarleton J, MacPherson J, Malmgren H, Dahl N, Barnicoat A, Mathew C, Mornet E, Tejada I, Maddalena A, Spiegel R, Schinzel A, Marcus J A G, Schwartz C, Mandel J L (1994). A multicenter study on genotype-phenotype correlations in the fragile X syndrome, using direct diagnosis with probe StB12.3: the first 2,253 cases. Am J Hum Genet, 55(2): 225–237
Spencer C M, Alekseyenko O, Hamilton S M, Thomas A M, Serysheva E, Yuva-Paylor L A, Paylor R (2011). Modifying behavioral phenotypes in Fmr1KO mice: genetic background differences reveal autistic-like responses. Autism Res, 4(1): 40–56
Srivastava D P, Woolfrey K M, Jones K A, Shum C Y, Lash L L, Swanson G T, Penzes P (2008). Rapid enhancement of two-step wiring plasticity by estrogen and NMDA receptor activity. Proc Natl Acad Sci USA, 105(38): 14650–14655
Tejada-Simon M V, Bongmba O T N (2012). Regulation of neuronal morphology and plasticity by small GTP-binding proteins: implications for autism and mental retardation disorders, in Horizons Neurosci. Res. (Andres Costa and Eugenio Villalba, ed.), pp. 1–67, Vol. 8, Ch.1, NOVA Sci. Pub., Hauppauge, NY
Thomas AM, Bui N, Graham D, Perkins J R, Yuva-Paylor L A, Paylor R (2011). Genetic reduction of group 1 metabotropic glutamate receptors alters select behaviors in a mouse model for fragile X syndrome. Behav Brain Res, 223(2): 310–321
Veeraragavan S, Bui N, Perkins J R, Yuva-Paylor L A, Carpenter R L, Paylor R (2011). Modulation of behavioral phenotypes by a muscarinic M1 antagonist in a mouse model of fragile X syndrome. Psychopharmacology (Berl), 217(1): 143–151
Woolley C S, Gould E, Frankfurt M, McEwen B S (1990). Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci, 10(12): 4035–4039
Woolley C S, McEwen B S (1992). Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci, 12(7): 2549–2554
Woolley C S, McEwen B S (1993). Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol, 336(2): 293–306
Yan Q J, Asafo-Adjei P K, Arnold H M, Brown R E, Bauchwitz R P (2004). A phenotypic and molecular characterization of the fmr1- tm1Cgr fragile X mouse. Genes Brain Behav, 3(6): 337–359
Zhao L, Brinton R D (2007). Estrogen receptor a and ß differentially regulate intracellular Ca(2 +) dynamics leading to ERK phosphorylation and estrogen neuroprotection in hippocampal neurons. Brain Res, 1172: 48–59
Zhao M G, Toyoda H, Ko S W, Ding H K, Wu L J, Zhuo M (2005). Deficits in trace fear memory and long-term potentiation in a mouse model for fragile X syndrome. J Neurosci, 25(32): 7385–7392
Zhao Z, Fan L, Fortress A M, Boulware M I, Frick K M (2012). Hippocampal histone acetylation regulates object recognition and the estradiol-induced enhancement of object recognition. J Neurosci, 32(7): 2344–2351
Zhao Z, Fan L, Frick K M (2010). Epigenetic alterations regulate estradiol-induced enhancement of memory consolidation. Proc Natl Acad Sci USA, 107(12): 5605–5610
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nguy, S., Tejada-Simon, M.V. Phenotype analysis and rescue on female FVB.129-Fmr1 knockout mice. Front. Biol. 11, 43–52 (2016). https://doi.org/10.1007/s11515-016-1391-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11515-016-1391-5