Abstract
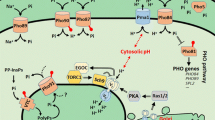
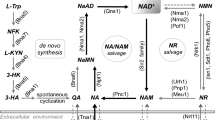
Nutrient sensing pathways and their regulation grant cells control over their metabolism and growth in response to changing nutrients. Factors that regulate nutrient sensing can also modulate longevity. Reduced activity of nutrient sensing pathways such as glucose-sensing PKA, nitrogen-sensing TOR and S6 kinase homolog Sch9 have been linked to increased life span in the yeast, Saccharomyces cerevisiae, and higher eukaryotes. Recently, reduced activity of amino acid sensing SPS pathway was also shown to increase yeast life span. Life span extension by reduced SPS activity requires enhanced NAD+ (nicotinamide adenine dinucleotide, oxidized form) and nicotinamide riboside (NR, a NAD+ precursor) homeostasis. Maintaining adequate NAD+ pools has been shown to play key roles in life span extension, but factors regulating NAD+ metabolism and homeostasis are not completely understood. Recently, NAD+ metabolism was also linked to the phosphate (Pi)-sensing PHO pathway in yeast. Canonical PHO activation requires Pi-starvation. Interestingly, NAD+ depletion without Pi-starvation was sufficient to induce PHO activation, increasing NR production and mobilization. Moreover, SPS signaling appears to function in parallel with PHO signaling components to regulate NR/NAD+ homeostasis. These studies suggest that NAD+ metabolism is likely controlled by and/or coordinated with multiple nutrient sensing pathways. Indeed, cross-regulation of PHO, PKA, TOR and Sch9 pathways was reported to potentially affect NAD+ metabolism; though detailed mechanisms remain unclear. This review discusses yeast longevity related nutrient sensing pathways and possible mechanisms of life span extension, regulation of NAD+ homeostasis, and cross-talk among nutrient sensing pathways and NAD+ homeostasis.
Similar content being viewed by others
References
Abdel-Sater F, Jean C, Merhi A, Vissers S, André B (2011). Amino acid signaling in yeast: activation of Ssy5 protease is associated with its phosphorylation-induced ubiquitylation. J Biol Chem, 286(14): 12006–12015
AbdelRaheim S R, Cartwright J L, Gasmi L, McLennan A G (2001). The NADH diphosphatase encoded by the Saccharomyces cerevisiae NPY1 nudix hydrolase gene is located in peroxisomes. Arch Biochem Biophys, 388(1): 18–24
Andersen M P, Nelson Z W, Hetrick E D, Gottschling D E (2008). A genetic screen for increased loss of heterozygosity in Saccharomyces cerevisiae. Genetics, 179(3): 1179–1195
Anderson R M, Bitterman K J, Wood J G, Medvedik O, Cohen H, Lin S S, Manchester J K, Gordon J I, Sinclair D A (2002). Manipulation of a nuclear NAD+ salvage pathway delays aging without altering steady-state NAD+ levels. J Biol Chem, 277(21): 18881–18890
Anderson R M, Bitterman K J, Wood J G, Medvedik O, Sinclair D A (2003). Nicotinamide and PNC1 govern lifespan extension by calorie restriction in Saccharomyces cerevisiae. Nature, 423(6936): 181–185
Andréasson C, Heessen S, Ljungdahl P O (2006). Regulation of transcription factor latency by receptor-activated proteolysis. Genes Dev, 20(12): 1563–1568
Ashrafi K, Lin S S, Manchester J K, Gordon J I (2000). Sip2p and its partner snf1p kinase affect aging in S. cerevisiae. Genes Dev, 14(15): 1872–1885
Auesukaree C, Homma T, Tochio H, Shirakawa M, Kaneko Y, Harashima S (2004). Intracellular phosphate serves as a signal for the regulation of the PHO pathway in Saccharomyces cerevisiae. J Biol Chem, 279(17): 17289–17294
Auesukaree C, Tochio H, Shirakawa M, Kaneko Y, Harashima S (2005). Plc1p, Arg82p, and Kcs1p, enzymes involved in inositol pyrophosphate synthesis, are essential for phosphate regulation and polypho-sphate accumulation in Saccharomyces cerevisiae. J Biol Chem, 280 (26): 25127–25133
Bakker B M, Overkamp K M, Kötter P, Luttik M A, Pronk J T, van Dijken J P, Pronk J T, and the van Maris AJ, and the van Dijken J P (2001). Stoichiometry and compartmentation of NADH metabolism in Saccharomyces cerevisiae. FEMS Microbiol Rev, 25(1): 15–37
Baldwin S A, Yao S Y, Hyde R J, Ng A M, Foppolo S, Barnes K, Ritzel M W, Cass C E, Young J D (2005). Functional characterization of novel human and mouse equilibrative nucleoside transporters (hENT3 and mENT3) located in intracellular membranes. J Biol Chem, 280(16): 15880–15887
Barros M H, Bandy B, Tahara E B, Kowaltowski A J (2004). Higher respiratory activity decreases mitochondrial reactive oxygen release and increases life span in Saccharomyces cerevisiae. J Biol Chem, 279(48): 49883–49888
Beck T, Hall M N (1999). The TOR signalling pathway controls nuclear localization of nutrient-regulated transcription factors. Nature, 402 (6762). 689–692
Bedalov A, Hirao M, Posakony J, Nelson M, Simon J A (2003). NAD+- dependent deacetylase Hst1p controls biosynthesis and cellular NAD+ levels in Saccharomyces cerevisiae. Mol Cell Biol, 23(19): 7044–7054
Belenky P, Racette F G, Bogan K L, McClure J M, Smith J S, Brenner C (2007). Nicotinamide riboside promotes Sir2 silencing and extends lifespan via Nrk and Urh1/Pnp1/Meu1 pathways to NAD+. Cell, 129 (3): 473–484
Belenky P A, Moga T G, Brenner C (2008). Saccharomyces cerevisiae YOR071C encodes the high affinity nicotinamide riboside transporter Nrt1. J Biol Chem, 283(13): 8075–8079
Bender D A (1983). Biochemistry of tryptophan in health and disease. Mol Aspects Med, 6(2): 101–197
Bieganowski P, Brenner C (2004). Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss-Handler independent route to NAD+ in fungi and humans. Cell, 117(4): 495–502
Bieganowski P, Pace H C, Brenner C (2003). Eukaryotic NAD+ synthetase Qns1 contains an essential, obligate intramolecular thiol glutamine amidotransferase domain related to nitrilase. J Biol Chem, 278(35): 33049–33055
Bilinski T, Bartosz G (2006). Hypothesis: cell volume limits cell divisions. Acta Biochim Pol, 53(4): 833–835
Bilinski T, Zadrag-Tecza R, Bartosz G (2012). Hypertrophy hypothesis as an alternative explanation of the phenomenon of replicative aging of yeast. FEMS Yeast Res, 12(1): 97–101
Binda M, Péli-Gulli M P, Bonfils G, Panchaud N, Urban J, Sturgill TW, Loewith R, De Virgilio C (2009). The Vam6 GEF controls TORC1 by activating the EGO complex. Mol Cell, 35(5): 563–573
Bitterman K J, Anderson R M, Cohen H Y, Latorre-Esteves M, Sinclair D A (2002). Inhibition of silencing and accelerated aging by nicotinamide, a putative negative regulator of yeast sir2 and human SIRT1. J Biol Chem, 277(47): 45099–45107
Blinder D, Coschigano P W, Magasanik B (1996). Interaction of the GATA factor Gln3p with the nitrogen regulator Ure2p in Saccharomyces cerevisiae. J Bacteriol, 178(15): 4734–4736
Bogan K L, Brenner C (2008). Nicotinic acid, nicotinamide, and nicotinamide riboside: a molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu Rev Nutr, 28(1): 115–130
Bogan K L, Evans C, Belenky P, Song P, Burant C F, Kennedy R, Brenner C (2009). Identification of Isn1 and Sdt1 as glucose- and vitamin-regulated nicotinamide mononucleotide and nicotinic acid mononucleotide [corrected] 5'-nucleotidases responsible for production of nicotinamide riboside and nicotinic acid riboside. J Biol Chem, 284(50): 34861–34869
Bonawitz N D, Chatenay-Lapointe M, Pan Y, Shadel G S (2007). Reduced TOR signaling extends chronological life span via increased respiration and upregulation of mitochondrial gene expression. Cell Metab, 5(4): 265–277
Boswell-Casteel R C, Johnson J M, Duggan K D, Roe-Žurž Z, Schmitz H, Burleson C, Hays F A (2014). FUN26 (function unknown now 26) protein from Saccharomyces cerevisiae is a broad selectivity, high affinity, nucleoside and nucleobase transporter. J Biol Chem, 289 (35): 24440–24451
Brachmann C B, Sherman J M, Devine S E, Cameron E E, Pillus L, Boeke J D (1995). The SIR2 gene family, conserved from bacteria to humans, functions in silencing, cell cycle progression, and chromosome stability. Genes Dev, 9(23): 2888–2902
Broach J R (2012). Nutritional control of growth and development in yeast. Genetics, 192(1): 73–105
Bun-Ya M, Nishimura M, Harashima S, Oshima Y (1991). The PHO84 gene of Saccharomyces cerevisiae encodes an inorganic phosphate transporter. Mol Cell Biol, 11(6): 3229–3238
Burtner C R, Murakami C J, Kennedy B K, Kaeberlein M (2009). A molecular mechanism of chronological aging in yeast. Cell Cycle, 8 (8): 1256–1270
Carroll A S, Bishop A C, DeRisi J L, Shokat K M, O’Shea E K (2001). Chemical inhibition of the Pho85 cyclin-dependent kinase reveals a role in the environmental stress response. Proc Natl Acad Sci USA, 98(22): 12578–12583
Casamayor A, Torrance P D, Kobayashi T, Thorner J, Alessi D R (1999). Functional counterparts of mammalian protein kinases PDK1 and SGK in budding yeast. Curr Biol, 9(4): 186–197
Celenza J L, Carlson M (1986). A yeast gene that is essential for release from glucose repression encodes a protein kinase. Science, 233 (4769). 1175–1180
Celic I, Masumoto H, Griffith W P, Meluh P, Cotter R J, Boeke J D, Verreault A (2006). The sirtuins hst3 and Hst4p preserve genome integrity by controlling histone h3 lysine 56 deacetylation. Curr Biol, 16(13): 1280–1289
Chandrashekarappa D G, McCartney R R, Schmidt M C (2013). Ligand binding to the AMP-activated protein kinase active site mediates protection of the activation loop from dephosphorylation. J Biol Chem, 288(1): 89–98
Cheng W, Roth J (1995). Isolation of NAD cycle mutants defective in nicotinamide mononucleotide deamidase in Salmonella typhimurium. J Bacteriol, 177(23): 6711–6717
Cherkasova V A, Hinnebusch A G (2003). Translational control by TOR and TAP42 through dephosphorylation of eIF2alpha kinase GCN2. Genes Dev, 17(7): 859–872
Chodosh L A, Olesen J, Hahn S, Baldwin A S, Guarente L, Sharp P A (1988). A yeast and a human CCAAT-binding protein have heterologous subunits that are functionally interchangeable. Cell, 53(1): 25–35
Choi KM, Kwon Y Y, Lee C K (2015). Disruption of Snf3/Rgt2 glucose sensors decreases lifespan and caloric restriction effectiveness through Mth1/Std1 by adjusting mitochondrial efficiency in yeast. FEBS Lett, 589(3): 349–357
Clapper D L, Walseth T F, Dargie P J, Lee H C (1987). Pyridine nucleotide metabolites stimulate calcium release from sea urchin egg microsomes desensitized to inositol trisphosphate. J Biol Chem, 262 (20): 9561–9568
Conrad M, Schothorst J, Kankipati H N, Van Zeebroeck G, Rubio- Texeira M, Thevelein J M (2014). Nutrient sensing and signaling in the yeast Saccharomyces cerevisiae. FEMS Microbiol Rev, 38(2): 254–299
De Wever V, Reiter W, Ballarini A, Ammerer G, Brocard C (2005). A dual role for PP1 in shaping the Msn2-dependent transcriptional response to glucose starvation. EMBO J, 24(23): 4115–4123
Delaney J R, Ahmed U, Chou A, Sim S, Carr D, Murakami C J, Schleit J, Sutphin G L, An E H, Castanza A, Fletcher M, Higgins S, Jelic M, Klum S, Muller B, Peng Z J, Rai D, Ros V, Singh M, Wende H V, Kennedy B K, Kaeberlein M (2013). Stress profiling of longevity mutants identifies Afg3 as a mitochondrial determinant of cytoplasmic mRNA translation and aging. Aging Cell, 12(1): 156–166
DeRisi J L, Iyer V R, Brown P O (1997). Exploring the metabolic and genetic control of gene expression on a genomic scale. Science, 278 (5338). 680–686
Dever T E, Hinnebusch A G (2005). GCN2 whets the appetite for amino acids. Mol Cell, 18(2): 141–142
Dilova I, Aronova S, Chen J C, Powers T (2004). Tor signaling and nutrient-based signals converge on Mks1p phosphorylation to regulate expression of Rtg1.Rtg3p-dependent target genes. J Biol Chem, 279(45): 46527–46535
Dilova I, Easlon E, Lin S J (2007). Calorie restriction and the nutrient sensing signaling pathways. Cell Mol Life Sci, 64(6): 752–767
Dohlman H G, Thorner J W (2001). Regulation of G protein-initiated signal transduction in yeast: paradigms and principles. Annu Rev Biochem, 70(1): 703–754
Dong J, Qiu H, Garcia-Barrio M, Anderson J, Hinnebusch A G (2000). Uncharged tRNA activates GCN2 by displacing the protein kinase moiety from a bipartite tRNA-binding domain. Mol Cell, 6(2): 269–279
Dubouloz F, Deloche O, Wanke V, Cameroni E, De Virgilio C (2005). The TOR and EGO protein complexes orchestrate microautophagy in yeast. Mol Cell, 19(1): 15–26
Easlon E, Tsang F, Dilova I, Wang C, Lu S P, Skinner C, Lin S J (2007). The dihydrolipoamide acetyltransferase is a novel metabolic longevity factor and is required for calorie restriction-mediated life span extension. J Biol Chem, 282(9): 6161–6171
Easlon E, Tsang F, Skinner C, Wang C, Lin S J (2008). The malateaspartate NADH shuttle components are novel metabolic longevity regulators required for calorie restriction-mediated life span extension in yeast. Genes Dev, 22(7): 931–944
Efeyan A, Zoncu R, Sabatini D M (2012). Amino acids and mTORC1: from lysosomes to disease. Trends Mol Med, 18(9): 524–533
Emanuelli M, Amici A, Carnevali F, Pierella F, Raffaelli N, Magni G (2003). Identification and characterization of a second NMN adenylyltransferase gene in Saccharomyces cerevisiae. Protein Expr Purif, 27(2): 357–364
Emanuelli M, Carnevali F, Lorenzi M, Raffaelli N, Amici A, Ruggieri S, Magni G (1999). Identification and characterization of YLR328W, the Saccharomyces cerevisiae structural gene encoding NMN adenylyltransferase. Expression and characterization of the recombinant enzyme. FEBS Lett, 455(1–2): 13–17
Endo Y, Obata T, Murata D, Ito M, Sakamoto K, Fukushima M, Yamasaki Y, Yamada Y, Natsume N, Sasaki T (2007). Cellular localization and functional characterization of the equilibrative nucleoside transporters of antitumor nucleosides. Cancer Sci, 98 (10): 1633–1637
Erjavec N, Bayot A, Gareil M, Camougrand N, Nystrom T, Friguet B, Bulteau A L (2013). Deletion of the mitochondrial Pim1/Lon protease in yeast results in accelerated aging and impairment of the proteasome. Free Radic Biol Med, 56: 9–16
Erjavec N, Cvijovic M, Klipp E, Nyström T (2008). Selective benefits of damage partitioning in unicellular systems and its effects on aging. Proc Natl Acad Sci USA, 105(48): 18764–18769
Erjavec N, Larsson L, Grantham J, Nyström T (2007). Accelerated aging and failure to segregate damaged proteins in Sir2 mutants can be suppressed by overproducing the protein aggregation-remodeling factor Hsp104p. Genes Dev, 21(19): 2410–2421
Erjavec N, Nyström T (2007). Sir2p-dependent protein segregation gives rise to a superior reactive oxygen species management in the progeny of Saccharomyces cerevisiae. Proc Natl Acad Sci USA, 104(26): 10877–10881
Eto K, Tsubamoto Y, Terauchi Y, Sugiyama T, Kishimoto T, Takahashi N, Yamauchi N, Kubota N, Murayama S, Aizawa T, Akanuma Y, Aizawa S, Kasai H, Yazaki Y, Kadowaki T (1999). Role of NADH shuttle system in glucose-induced activation of mitochondrial metabolism and insulin secretion. Science, 283(5404): 981–985
Evans C, Bogan K L, Song P, Burant C F, Kennedy R T, Brenner C (2010). NAD+ metabolite levels as a function of vitamins and calorie restriction: evidence for different mechanisms of longevity. BMC Chem Biol, 10(1): 2
Fabrizio P, Gattazzo C, Battistella L, Wei M, Cheng C, McGrew K, Longo V D (2005). Sir2 blocks extreme life-span extension. Cell, 123 (4): 655–667
Fabrizio P, Hoon S, Shamalnasab M, Galbani A, Wei M, Giaever G, Nislow C, Longo V D (2010). Genome-wide screen in Saccharomyces cerevisiae identifies vacuolar protein sorting, autophagy, biosynthetic, and tRNA methylation genes involved in life span regulation. PLoS Genet, 6(7): e1001024
Fabrizio P, Longo V D (2003). The chronological life span of Saccharomyces cerevisiae. Aging Cell, 2(2): 73–81
Fabrizio P, Longo V D (2007). The chronological life span of Saccharomyces cerevisiae. Methods Mol Biol, 371: 89–95
Fabrizio P, Pozza F, Pletcher S D, Gendron C M, Longo V D (2001). Regulation of longevity and stress resistance by Sch9 in yeast. Science, 292(5515): 288–290
Flick K M, Spielewoy N, Kalashnikova T I, Guaderrama M, Zhu Q, Chang H C, Wittenberg C (2003). Grr1-dependent inactivation of Mth1 mediates glucose-induced dissociation of Rgt1 from HXT gene promoters. Mol Biol Cell, 14(8): 3230–3241
Foresti O, Rodriguez-Vaello V, Funaya C, Carvalho P (2014). Quality control of inner nuclear membrane proteins by the Asi complex. Science, 346(6210): 751–755
Forsburg S L, Guarente L (1989). Identification and characterization of HAP4: a third component of the CCAAT-bound HAP2/HAP3 heteromer. Genes Dev, 3(8): 1166–1178
Frye R A (2000). Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem Biophys Res Commun, 273 (2): 793–798
Gallo C M, Smith D L Jr, Smith J S (2004). Nicotinamide clearance by Pnc1 directly regulates Sir2-mediated silencing and longevity. Mol Cell Biol, 24(3): 1301–1312
Gancedo J M (1998). Yeast carbon catabolite repression. Microbiol Mol Biol Rev, 62(2): 334–361
Garavaglia S, D’Angelo I, Emanuelli M, Carnevali F, Pierella F, Magni G, Rizzi M (2002). Structure of human NMN adenylyltransferase. A key nuclear enzyme for NAD homeostasis. J Biol Chem, 277(10): 8524–8530
Gauthier S, Coulpier F, Jourdren L, Merle M, Beck S, Konrad M, Daignan-Fornier B, Pinson B (2008). Co-regulation of yeast purine and phosphate pathways in response to adenylic nucleotide variations. Mol Microbiol, 68(6): 1583–1594
Ghislain M, Talla E, François J M (2002). Identification and functional analysis of the Saccharomyces cerevisiae nicotinamidase gene, PNC1. Yeast, 19(3): 215–224
Giots F, Donaton M C, Thevelein J M (2003). Inorganic phosphate is sensed by specific phosphate carriers and acts in concert with glucose as a nutrient signal for activation of the protein kinase A pathway in the yeast Saccharomyces cerevisiae. Mol Microbiol, 47(4): 1163–1181
Godard P, Urrestarazu A, Vissers S, Kontos K, Bontempi G, van Helden J, André B (2007). Effect of 21 different nitrogen sources on global gene expression in the yeast Saccharomyces cerevisiae. Mol Cell Biol, 27(8): 3065–3086
Görner W, Durchschlag E, Martinez-Pastor MT, Estruch F, Ammerer G, Hamilton B, Ruis H, Schüller C (1998). Nuclear localization of the C2H2 zinc finger protein Msn2p is regulated by stress and protein kinase A activity. Genes Dev, 12(4): 586–597
Görner W, Durchschlag E, Wolf J, Brown E L, Ammerer G, Ruis H, Schüller C (2002). Acute glucose starvation activates the nuclear localization signal of a stress-specific yeast transcription factor. EMBO J, 21(1–2): 135–144
Graeff R, Liu Q, Kriksunov I A, Hao Q, Lee H C (2006). Acidic residues at the active sites of CD38 and ADP-ribosyl cyclase determine nicotinic acid adenine dinucleotide phosphate (NAADP) synthesis and hydrolysis activities. J Biol Chem, 281(39): 28951–28957
Grose J H, Bergthorsson U, Roth J R (2005). Regulation of NAD synthesis by the trifunctional NadR protein of Salmonella enterica. J Bacteriol, 187(8): 2774–2782
Guarente L (2013). Introduction: sirtuins in aging and diseases. Methods Mol Biol, 1077: 3–10
Guse A H, Lee H C (2008). NAADP: a universal Ca2+ trigger. Sci Signal, 1(44): re10
Hachinohe M, Hanaoka F, Masumoto H (2011). Hst3 and Hst4 histone deacetylases regulate replicative lifespan by preventing genome instability in Saccharomyces cerevisiae. Genes Cells, 16(4): 467–477
Hachinohe M, Yamane M, Akazawa D, Ohsawa K, Ohno M, Terashita Y, Masumoto H (2013). A reduction in age-enhanced gluconeogenesis extends lifespan. PLoS ONE, 8(1): e54011
Hahn J S, Thiele D J (2004). Activation of the Saccharomyces cerevisiae heat shock transcription factor under glucose starvation conditions by Snf1 protein kinase. J Biol Chem, 279(7): 5169–5176
Hahn S, Guarente L (1988). Yeast HAP2 and HAP3: transcriptional activators in a heteromeric complex. Science, 240(4850): 317–321
Hahn S, Young E T (2011). Transcriptional regulation in Saccharomyces cerevisiae: transcription factor regulation and function, mechanisms of initiation, and roles of activators and coactivators. Genetics, 189 (3): 705–736
Haigis M C, Mostoslavsky R, Haigis K M, Fahie K, Christodoulou D C, Murphy A J, Valenzuela D M, Yancopoulos G D, Karow M, Blander G, Wolberger C, Prolla T A, Weindruch R, Alt F W, Guarente L (2006). SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell, 126(5): 941–954
Halme A, Bumgarner S, Styles C, Fink G R (2004). Genetic and epigenetic regulation of the FLO gene family generates cell-surface variation in yeast. Cell, 116(3): 405–415
Hardie D G (2007). AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol, 8(10): 774–785
Hecht A, Strahl-Bolsinger S, Grunstein M (1996). Spreading of transcriptional repressor SIR3 from telomeric heterochromatin. Nature, 383(6595): 92–96
Hernández H, Aranda C, López G, Riego L, González A (2011). Hap2-3- 5-Gln3 determine transcriptional activation of GDH1 and ASN1 under repressive nitrogen conditions in the yeast Saccharomyces cerevisiae. Microbiology, 157(Pt 3): 879–889
Hinnebusch A G (2005). Translational regulation of GCN4 and the general amino acid control of yeast. Annu Rev Microbiol, 59(1): 407–450
Hinnebusch A G, Natarajan K (2002). Gcn4p, a master regulator of gene expression, is controlled at multiple levels by diverse signals of starvation and stress. Eukaryot Cell, 1(1): 22–32
Hong S P, Leiper F C, Woods A, Carling D, Carlson M (2003). Activation of yeast Snf1 and mammalian AMP-activated protein kinase by upstream kinases. Proc Natl Acad Sci USA, 100(15): 8839–8843
Houtkooper R H, Cantó C, Wanders R J, Auwerx J (2010). The secret life of NAD+: an old metabolite controlling new metabolic signaling pathways. Endocr Rev, 31(2): 194–223
Hughes A L, Gottschling D E (2012). An early age increase in vacuolar pH limits mitochondrial function and lifespan in yeast. Nature, 492 (7428). 261–265
Imai S, Armstrong C M, Kaeberlein M, Guarente L (2000). Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature, 403(6771): 795–800
Imai S I, Guarente L (2014). NAD and sirtuins in aging and disease. Trends Cell Biol.
Ivy JM, Klar A J, Hicks J B (1986). Cloning and characterization of four SIR genes of Saccharomyces cerevisiae. Mol Cell Biol, 6: 688–702
Jacinto E, Lorberg A (2008). TOR regulation of AGC kinases in yeast and mammals. Biochem J, 410(1): 19–37
Jazwinski S M (1990). An experimental system for the molecular analysis of the aging process: the budding yeast Saccharomyces cerevisiae. J Gerontol, 45(3): B68–B74
Jazwinski SM(2000). Aging and longevity genes. Acta Biochim Pol, 47 (2): 269–279
Jia S H, Li Y, Parodo J, Kapus A, Fan L, Rotstein O D, Marshall J C (2004). Pre-B cell colony-enhancing factor inhibits neutrophil apoptosis in experimental inflammation and clinical sepsis. J Clin Invest, 113(9): 1318–1327
Jiang J C, Jaruga E, Repnevskaya M V, Jazwinski S M (2000). An intervention resembling caloric restriction prolongs life span and retards aging in yeast. FASEB J, 14(14): 2135–2137
Jouandot D, Roy A, Kim J H (2011). Functional dissection of the glucose signaling pathways that regulate the yeast glucose transporter gene (HXT) repressor Rgt1. J Cell Biochem, 112(11): 3268–3275
Kaeberlein M, Andalis A A, Fink G R, Guarente L (2002). High osmolarity extends life span in Saccharomyces cerevisiae by a mechanism related to calorie restriction. Mol Cell Biol, 22(22): 8056–8066
Kaeberlein M, Hu D, Kerr E O, Tsuchiya M, Westman E A, Dang N, Fields S, Kennedy B K (2005a). Increased life span due to calorie restriction in respiratory-deficient yeast. PLoS Genet, 1(5): e69
Kaeberlein M, Kirkland K T, Fields S, Kennedy B K (2004). Sir2- independent life span extension by calorie restriction in yeast. PLoS Biol, 2(9): e296
Kaeberlein M, Powers RW, Steffen K K, Westman E A, Hu D, Dang N, Kerr E O, Kirkland K T, Fields S, Kennedy B K (2005b). Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science, 310(5751): 1193–1196
Kamada Y, Fujioka Y, Suzuki N N, Inagaki F, Wullschleger S, Loewith R, Hall M N, Ohsumi Y (2005). Tor2 directly phosphorylates the AGC kinase Ypk2 to regulate actin polarization. Mol Cell Biol, 25 (16): 7239–7248
Kang H J, Jeong S J, Kim K N, Baek I J, Chang M, Kang CM, Park Y S, Yun C W (2014). A novel protein, Pho92, has a conserved YTH domain and regulates phosphate metabolism by decreasing the mRNA stability of PHO4 in Saccharomyces cerevisiae. Biochem J, 457(3): 391–400
Kato M, Lin S J (2014a). Regulation of NAD+ metabolism, signaling and compartmentalization in the yeast Saccharomyces cerevisiae. DNA Repair (Amst), 23: 49–58
Kato M, Lin S J (2014b). YCL047C/POF1 is a novel nicotinamide mononucleotide adenylyltransferase (NMNAT) in Saccharomyces cerevisiae. J Biol Chem, 289(22): 15577–15587
Keith C T, Schreiber S L (1995). PIK-related kinases: DNA repair, recombination, and cell cycle checkpoints. Science, 270(5233): 50–51
Kenyon C (2001). A conserved regulatory system for aging. Cell, 105 (2): 165–168
Kim J H, Brachet V, Moriya H, Johnston M (2006). Integration of transcriptional and posttranslational regulation in a glucose signal transduction pathway in Saccharomyces cerevisiae. Eukaryot Cell, 5 (1): 167–173
Kim J H, Johnston M (2006). Two glucose-sensing pathways converge on Rgt1 to regulate expression of glucose transporter genes in Saccharomyces cerevisiae. J Biol Chem, 281(36): 26144–26149
Kornitzer D, Raboy B, Kulka R G, Fink G R (1994). Regulated degradation of the transcription factor Gcn4. EMBO J, 13(24): 6021–6030
Kruegel U, Robison B, Dange T, Kahlert G, Delaney J R, Kotireddy S, Tsuchiya M, Tsuchiyama S, Murakami C J, Schleit J, Sutphin G, Carr D, Tar K, Dittmar G, Kaeberlein M, Kennedy B K, Schmidt M (2011). Elevated proteasome capacity extends replicative lifespan in Saccharomyces cerevisiae. PLoS Genet, 7(9): e1002253
Lamming DW, Latorre-Esteves M, Medvedik O, Wong S N, Tsang F A, Wang C, Lin S J, Sinclair D A (2005). HST2 mediates SIR2- independent life-span extension by calorie restriction. Science, 309 (5742). 1861–1864
Lamming D W, Wood J G, Sinclair D A (2004). Small molecules that regulate lifespan: evidence for xenohormesis. Mol Microbiol, 53(4): 1003–1009
Landry J, Sutton A, Tafrov S T, Heller R C, Stebbins J, Pillus L, Sternglanz R (2000). The silencing protein SIR2 and its homologs are NAD-dependent protein deacetylases. Proc Natl Acad Sci USA, 97 (11): 5807–5811
Lascaris R, Bussemaker H J, Boorsma A, Piper M, van der Spek H, Grivell L, Blom J (2003). Hap4p overexpression in glucose-grown Saccharomyces cerevisiae induces cells to enter a novel metabolic state. Genome Biol, 4(1): R3
Lee P, Kim MS, Paik SM, Choi S H, Cho B R, Hahn J S (2013). Rim15- dependent activation of Hsf1 and Msn2/4 transcription factors by direct phosphorylation in Saccharomyces cerevisiae. FEBS Lett, 587 (22): 3648–3655
Lee Y S, Huang K, Quiocho F A, O’Shea E K (2008). Molecular basis of cyclin-CDK-CKI regulation by reversible binding of an inositol pyrophosphate. Nat Chem Biol, 4(1): 25–32
Lee Y S, Mulugu S, York J D, O’Shea E K (2007). Regulation of a cyclin-CDK-CDK inhibitor complex by inositol pyrophosphates. Science, 316(5821): 109–112
Lenburg M E, O’Shea E K (1996). Signaling phosphate starvation. Trends Biochem Sci, 21(10): 383–387
Lewis C A, Parker S J, Fiske B P, McCloskey D, Gui D Y, Green C R, Vokes N I, Feist A M, Vander Heiden M G, Metallo C M (2014). Tracing compartmentalized NADPH metabolism in the cytosol and mitochondria of mammalian cells. Mol Cell, 55(2): 253–263
Li B, Skinner C, Castello P R, Kato M, Easlon E, Xie L, Li T, Lu S P, Wang C, Tsang F, Poyton R O, Lin S J (2011). Identification of potential calorie restriction-mimicking yeast mutants with increased mitochondrial respiratory chain and nitric oxide levels. J Aging Res, 2011: 673185
Li M, Valsakumar V, Poorey K, Bekiranov S, Smith J S (2013). Genome-wide analysis of functional sirtuin chromatin targets in yeast. Genome Biol, 14(5): R48
Li P L, Zhang Y, Abais J M, Ritter J K, Zhang F (2013). Cyclic ADPribose and NAADP in vascular regulation and diseases. Messenger (Los Angel), 2(2): 63–85
Lin S J, Defossez P A, Guarente L (2000). Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science, 289(5487): 2126–2128
Lin S J, Ford E, Haigis M, Liszt G, Guarente L (2004). Calorie restriction extends yeast life span by lowering the level of NADH. Genes Dev, 18(1): 12–16
Lin S J, Kaeberlein M, Andalis A A, Sturtz L A, Defossez P A, Culotta V C, Fink G R, Guarente L (2002). Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature, 418(6895): 344–348
Lin S S, Manchester J K, Gordon J I (2003). Sip2, an N-myristoylated beta subunit of Snf1 kinase, regulates aging in Saccharomyces cerevisiae by affecting cellular histone kinase activity, recombination at rDNA loci, and silencing. J Biol Chem, 278(15): 13390–13397
Lin Y Y, Lu J Y, Zhang J, Walter W, Dang W, Wan J, Tao S C, Qian J, Zhao Y, Boeke J D, Berger S L, Zhu H (2009). Protein acetylation microarray reveals that NuA4 controls key metabolic target regulating gluconeogenesis. Cell, 136(6): 1073–1084
Liu Z, Thornton J, Spírek M, Butow R A (2008). Activation of the SPS amino acid-sensing pathway in Saccharomyces cerevisiae correlates with the phosphorylation state of a sensor component, Ptr3. Mol Cell Biol, 28(2): 551–563
Ljungdahl P O (2009). Amino-acid-induced signalling via the SPSsensing pathway in yeast. Biochem Soc Trans, 37(Pt 1): 242–247
Ljungdahl P O, Daignan-Fornier B (2012). Regulation of amino acid, nucleotide, and phosphate metabolism in Saccharomyces cerevisiae. Genetics, 190(3): 885–929
Llorente B, Dujon B (2000). Transcriptional regulation of the Saccharomyces cerevisiae DAL5 gene family and identification of the high affinity nicotinic acid permease TNA1 (YGR260w). FEBS Lett, 475(3): 237–241
Loewith R, Jacinto E, Wullschleger S, Lorberg A, Crespo J L, Bonenfant D, Oppliger W, Jenoe P, Hall M N (2002). Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell, 10(3): 457–468
Longo V D (2003). The Ras and Sch9 pathways regulate stress resistance and longevity. Exp Gerontol, 38(7): 807–811
Longo V D, Fabrizio P (2012). Chronological aging in Saccharomyces cerevisiae. Subcell Biochem, 57: 101–121
Lu J Y, Lin Y Y, Sheu J C, Wu J T, Lee F J, Chen Y, Lin M I, Chiang F T, Tai T Y, Berger S L, Zhao Y, Tsai K S, Zhu H, Chuang L M, Boeke J D (2011). Acetylation of yeast AMPK controls intrinsic aging independently of caloric restriction. Cell, 146(6): 969–979
Lu S P, Kato M, Lin S J (2009). Assimilation of endogenous nicotinamide riboside is essential for calorie restriction-mediated life span extension in Saccharomyces cerevisiae. J Biol Chem, 284 (25): 17110–17119
Lu S P, Lin S J (2010). Regulation of yeast sirtuins by NAD(+) metabolism and calorie restriction. Biochim Biophys Acta, 1804(8): 1567–1575
Lu S P, Lin S J (2011). Phosphate-responsive signaling pathway is a novel component of NAD+ metabolism in Saccharomyces cerevisiae. J Biol Chem, 286(16): 14271–14281
Lundh F, Mouillon J M, Samyn D, Stadler K, Popova Y, Lagerstedt J O, Thevelein J M, Persson B L (2009). Molecular mechanisms controlling phosphate-induced downregulation of the yeast Pho84 phosphate transporter. Biochemistry, 48(21): 4497–4505
Magni G, Amici A, Emanuelli M, Orsomando G, Raffaelli N, Ruggieri S (2004). Structure and function of nicotinamide mononucleotide adenylyltransferase. Curr Med Chem, 11(7): 873–885
Marzluf G A (1997). Molecular genetics of sulfur assimilation in filamentous fungi and yeast. Annu Rev Microbiol, 51(1): 73–96
Matecic M, Smith D L, Pan X, Maqani N, Bekiranov S, Boeke J D, Smith J S (2010). A microarray-based genetic screen for yeast chronological aging factors. PLoS Genet, 6(4): e1000921
Mayer F V, Heath R, Underwood E, Sanders M J, Carmena D, McCartney R R, Leiper F C, Xiao B, Jing C, Walker P A, Haire L F, Ogrodowicz R, Martin S R, Schmidt M C, Gamblin S J, Carling D (2011). ADP regulates SNF1, the Saccharomyces cerevisiae homolog of AMP-activated protein kinase. Cell Metab, 14(5): 707–714
McCartney R R, Schmidt M C (2001). Regulation of Snf1 kinase. ACTIVATION REQUIRES PHOSPHORYLATION OF THREONINE 210 BY AN UPSTREAM KINASE AS WELL AS A DISTINCT STEP MEDIATED BY THE SNF4 SUBUNIT. J Biol Chem, 276(39): 36460–36466
McNabb D S, Pinto I (2005). Assembly of the Hap2p/Hap3p/Hap4p/ Hap5p-DNA complex in Saccharomyces cerevisiae. Eukaryot Cell, 4 (11): 1829–1839
McNabb D S, Xing Y, Guarente L (1995). Cloning of yeast HAP5: a novel subunit of a heterotrimeric complex required for CCAAT binding. Genes Dev, 9(1): 47–58
Medvedik O, Lamming DW, Kim K D, Sinclair D A (2007). MSN2 and MSN4 link calorie restriction and TOR to sirtuin-mediated lifespan extension in Saccharomyces cerevisiae. PLoS Biol, 5(10): e261
Menoyo S, Ricco N, Bru S, Hernández-Ortega S, Escoté X, Aldea M, Clotet J (2013). Phosphate-activated cyclin-dependent kinase stabilizes G1 cyclin to trigger cell cycle entry. Mol Cell Biol, 33 (7): 1273–1284
Mense S M, Zhang L (2006). Heme: a versatile signaling molecule controlling the activities of diverse regulators ranging from transcription factors to MAP kinases. Cell Res, 16(8): 681–692
Mesquita A, Weinberger M, Silva A, Sampaio-Marques B, Almeida B, Leão C, Costa V, Rodrigues F, Burhans W C, Ludovico P (2010). Caloric restriction or catalase inactivation extends yeast chronological lifespan by inducing H2O2 and superoxide dismutase activity. Proc Natl Acad Sci USA, 107(34): 15123–15128
Moazed D (2001). Common themes in mechanisms of gene silencing. Mol Cell, 8(3): 489–498
Moriya H, Johnston M (2004). Glucose sensing and signaling in Saccharomyces cerevisiae through the Rgt2 glucose sensor and casein kinase I. Proc Natl Acad Sci USA, 101(6): 1572–1577
Mortimer R K, Johnston J R (1959). Life span of individual yeast cells. Nature, 183(4677): 1751–1752
Mouillon J M, Persson B L (2005). Inhibition of the protein kinase A alters the degradation of the high-affinity phosphate transporter Pho84 in Saccharomyces cerevisiae. Curr Genet, 48(4): 226–234
Murakami C, Delaney J R, Chou A, Carr D, Schleit J, Sutphin G L, An E H, Castanza A S, Fletcher M, Goswami S, Higgins S, Holmberg M, Hui J, Jelic M, Jeong K S, Kim J R, Klum S, Liao E, Lin M S, Lo W, Miller H, Moller R, Peng Z J, Pollard T, Pradeep P, Pruett D, Rai D, Ros V, Schuster A, Singh M, Spector B L, VanderWende H, Wang A M, Wasko B M, Olsen B, Kaeberlein M (2012). pH neutralization protects against reduction in replicative lifespan following chronological aging in yeast. Cell Cycle, 11(16): 3087–3096
Murakami C J, Wall V, Basisty N, Kaeberlein M (2011). Composition and acidification of the culture medium influences chronological aging similarly in vineyard and laboratory yeast. PLoS ONE, 6(9): e24530
Natalini P, Ruggieri S, Raffaelli N, Magni G (1986). Nicotinamide mononucleotide adenylyltransferase. Molecular and enzymatic properties of the homogeneous enzyme from baker’s yeast. Biochemistry, 25(12): 3725–3729
Natarajan K, Meyer MR, Jackson BM, Slade D, Roberts C, Hinnebusch A G, Marton M J (2001). Transcriptional profiling shows that Gcn4p is a master regulator of gene expression during amino acid starvation in yeast. Mol Cell Biol, 21(13): 4347–4368
Niles B J, Powers T (2014). TOR complex 2-Ypk1 signaling regulates actin polarization via reactive oxygen species. Mol Biol Cell, 25(24): 3962–3972
Noda T, Klionsky D J (2008). The quantitative Pho8Delta60 assay of nonspecific autophagy. Methods Enzymol, 451: 33–42
North B J, Verdin E (2004). Sirtuins: Sir2-related NAD-dependent protein deacetylases. Genome Biol, 5(5): 224
Ocampo A, Liu J, Barrientos A (2013). NAD+ salvage pathway proteins suppress proteotoxicity in yeast models of neurodegeneration by promoting the clearance of misfolded/oligomerized proteins. Hum Mol Genet, 22(9): 1699–1708
Ocampo A, Liu J, Schroeder E A, Shadel G S, Barrientos A (2012). Mitochondrial respiratory thresholds regulate yeast chronological life span and its extension by caloric restriction. Cell Metab, 16(1): 55–67
Ohashi K, Kawai S, Murata K (2013). Secretion of quinolinic acid, an intermediate in the kynurenine pathway, for utilization in NAD+ biosynthesis in the yeast Saccharomyces cerevisiae. Eukaryot Cell, 12(5): 648–653
Omnus D J, Ljungdahl P O (2014). Latency of transcription factor Stp1 depends on a modular regulatory motif that functions as cytoplasmic retention determinant and nuclear degron. Mol Biol Cell, 25(23): 3823–3833
Omnus D J, Pfirrmann T, Andréasson C, Ljungdahl P O (2011). A phosphodegron controls nutrient-induced proteasomal activation of the signaling protease Ssy5. Mol Biol Cell, 22(15): 2754–2765
Overton M C, Chinault S L, Blumer K J (2005). Oligomerization of Gprotein- coupled receptors: lessons from the yeast Saccharomyces cerevisiae. Eukaryot Cell, 4(12): 1963–1970
Pan Y (2011). Mitochondria, reactive oxygen species, and chronological aging: a message from yeast. Exp Gerontol, 46(11): 847–852
Pan Y, Schroeder E A, Ocampo A, Barrientos A, Shadel G S (2011). Regulation of yeast chronological life span by TORC1 via adaptive mitochondrial ROS signaling. Cell Metab, 13(6): 668–678
Panozzo C, Nawara M, Suski C, Kucharczyka R, Skoneczny M, Bécam A M, Rytka J, Herbert C J (2002). Aerobic and anaerobic NAD+ metabolism in Saccharomyces cerevisiae. FEBS Lett, 517(1–3): 97–102
Parua P K, Ratnakumar S, Braun K A, Dombek K M, Arms E, Ryan P M, Young E T (2010). 14-3-3 (Bmh) proteins inhibit transcription activation by Adr1 through direct binding to its regulatory domain. Mol Cell Biol, 30(22): 5273–5283
Pasula S, Jouandot D, Kim J H (2007). Biochemical evidence for glucose-independent induction of HXT expression in Saccharomyces cerevisiae. FEBS Lett, 581(17): 3230–3234
Peeters T, Louwet W, Geladé R, Nauwelaers D, Thevelein J M, Versele M (2006). Kelch-repeat proteins interacting with the Ga protein Gpa2 bypass adenylate cyclase for direct regulation of protein kinase A in yeast. Proc Natl Acad Sci USA, 103(35): 13034–13039
Perrod S, Cockell MM, Laroche T, Renauld H, Ducrest A L, Bonnard C, Gasser S M (2001). A cytosolic NAD-dependent deacetylase, Hst2p, can modulate nucleolar and telomeric silencing in yeast. EMBO J, 20 (1–2): 197–209
Persson B L, Lagerstedt J O, Pratt J R, Pattison-Granberg J, Lundh K, Shokrollahzadeh S, Lundh F (2003). Regulation of phosphate acquisition in Saccharomyces cerevisiae. Curr Genet, 43(4): 225–244
Pinson B, Vaur S, Sagot I, Coulpier F, Lemoine S, Daignan-Fornier B (2009). Metabolic intermediates selectively stimulate transcription factor interaction and modulate phosphate and purine pathways. Genes Dev, 23(12): 1399–1407
Popova Y, Thayumanavan P, Lonati E, Agrochão M, Thevelein J M (2010). Transport and signaling through the phosphate-binding site of the yeast Pho84 phosphate transceptor. Proc Natl Acad Sci USA, 107(7): 2890–2895
Powers R W, Kaeberlein M, Caldwell S D, Kennedy B K, Fields S (2006). Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes Dev, 20(2): 174–184
Preiss J, Handler P (1958a). Biosynthesis of diphosphopyridine nucleotide. I. Identification of intermediates. J Biol Chem, 233(2): 488–492
Preiss J, Handler P (1958b). Biosynthesis of diphosphopyridine nucleotide. II. Enzymatic aspects. J Biol Chem, 233(2): 493–500
Ramsey K M, Mills K F, Satoh A, Imai S (2008). Age-associated loss of Sirt1-mediated enhancement of glucose-stimulated insulin secretion in beta cell-specific Sirt1-overexpressing (BESTO) mice. Aging Cell, 7(1): 78–88
Revollo J R, Grimm A A, Imai S (2004). The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyl transferase regulates Sir2 activity in mammalian cells. J Biol Chem, 279(49): 50754–50763
Rine J, Herskowitz I (1987). Four genes responsible for a position effect on expression from HML and HMR in Saccharomyces cerevisiae. Genetics, 116(1): 9–22
Rodgers J T, Lerin C, Haas W, Gygi S P, Spiegelman B M, Puigserver P (2005). Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature, 434(7029): 113–118
Rolland F, De Winde J H, Lemaire K, Boles E, Thevelein J M, Winderickx J (2000). Glucose-induced cAMP signalling in yeast requires both a G-protein coupled receptor system for extracellular glucose detection and a separable hexose kinase-dependent sensing process. Mol Microbiol, 38(2): 348–358
Roosen J, Engelen K, Marchal K, Mathys J, Griffioen G, Cameroni E, Thevelein JM, De Virgilio C, De Moor B, Winderickx J (2005). PKA and Sch9 control a molecular switch important for the proper adaptation to nutrient availability. Mol Microbiol, 55(3): 862–880
Roth S, Kumme J, Schüller H J (2004). Transcriptional activators Cat8 and Sip4 discriminate between sequence variants of the carbon source-responsive promoter element in the yeast Saccharomyces cerevisiae. Curr Genet, 45(3): 121–128
Rubenstein E M, McCartney R R, Zhang C, Shokat K M, Shirra M K, Arndt K M, Schmidt M C (2008). Access denied: Snf1 activation loop phosphorylation is controlled by availability of the phosphorylated threonine 210 to the PP1 phosphatase. J Biol Chem, 283(1): 222–230
Rubio-Texeira M, Van Zeebroeck G, Voordeckers K, Thevelein J M (2010). Saccharomyces cerevisiae plasma membrane nutrient sensors and their role in PKA signaling. FEMS Yeast Res, 10(2): 134–149
Samyn D R, Ruiz-Pávon L, Andersson M R, Popova Y, Thevelein J M, Persson B L (2012). Mutational analysis of putative phosphate- and proton-binding sites in the Saccharomyces cerevisiae Pho84 phosphate:H(+) transceptor and its effect on signalling to the PKA and PHO pathways. Biochem J, 445(3): 413–422
Sancak Y, Peterson T R, Shaul Y D, Lindquist R A, Thoreen C C, Bar-Peled L, Sabatini D M (2008). The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science, 320(5882): 1496–1501
Sanz P (2003). Snf1 protein kinase: a key player in the response to cellular stress in yeast. Biochem Soc Trans, 31(Pt 1): 178–181
Sasaki Y, Araki T, Milbrandt J (2006). Stimulation of nicotinamide adenine dinucleotide biosynthetic pathways delays axonal degeneration after axotomy. J Neurosci, 26(33): 8484–8491
Sauve A A, Schramm V L (2003). Sir2 regulation by nicotinamide results from switching between base exchange and deacetylation chemistry. Biochemistry, 42(31): 9249–9256
Scheckhuber C Q, Erjavec N, Tinazli A, Hamann A, Nyström T, Osiewacz H D (2007). Reducing mitochondrial fission results in increased life span and fitness of two fungal ageing models. Nat Cell Biol, 9(1): 99–105
Schleit J, Johnson S C, Bennett C F, Simko M, Trongtham N, Castanza A, Hsieh E J, Moller R M, Wasko B M, Delaney J R, Sutphin G L, Carr D, Murakami C J, Tocchi A, Xian B, Chen W, Yu T, Goswami S, Higgins S, Jeong K S, Kim J R, Klum S, Liao E, Lin M S, Lo W, Miller H, Olsen B, Peng Z J, Pollard T, Pradeep P, Pruett D, Rai D, Ros V, Singh M, Spector B L,We nde H V, An E H, Fletcher M, Jelic M, Rabinovitch P S, Maccoss M J, Han J D, Kennedy B K, Kaeberlein M (2013). Molecular mechanisms underlying genotypedependent responses to dietary restriction. Aging Cell, 12(6): 1050–1061
Schmeisser K, Mansfeld J, Kuhlow D, Weimer S, Priebe S, Heiland I, Birringer M, Groth M, Segref A, Kanfi Y, Price N L, Schmeisser S, Schuster S, Pfeiffer A F, Guthke R, Platzer M, Hoppe T, Cohen H Y, Zarse K, Sinclair D A, Ristow M, Klum S, Liao E, Lin M S, Lo W, Miller H, Olsen B, Peng Z J, Pollard T, Pradeep P, Pruett D, Rai D, Ros V, Singh M, Spector B L,We nde H V, An E H, Fletcher M, Jelic M, Rabinovitch P S, Maccoss M J, Han J D, Kennedy B K, Kaeberlein M (2013). Role of sirtuins in lifespan regulation is linked to methylation of nicotinamide. Nat Chem Biol, 9(11): 693–700
Schmidt M T, Smith B C, Jackson M D, Denu J M (2004). Coenzyme specificity of Sir2 protein deacetylases: implications for physiological regulation. J Biol Chem, 279(38): 40122–40129
Schmidt-Brauns J, Herbert M, Kemmer G, Kraiss A, Schlör S, Reidl J (2001). Is a NAD pyrophosphatase activity necessary for Haemophilus influenzae type b multiplication in the blood stream? Int J Med Microbiol, 291(3): 219–225
Schüller H J (2003). Transcriptional control of nonfermentative metabolism in the yeast Saccharomyces cerevisiae. Curr Genet, 43 (3): 139–160
Schulz T J, Zarse K, Voigt A, Urban N, Birringer M, Ristow M (2007). Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab, 6(4): 280–293
Shama S, Lai C Y, Antoniazzi J M, Jiang J C, Jazwinski S M (1998). Heat stress-induced life span extension in yeast. Exp Cell Res, 245 (2): 379–388
Shimada K, Filipuzzi I, Stahl M, Helliwell S B, Studer C, Hoepfner D, Seeber A, Loewith R, Movva N R, Gasser S M (2013). TORC2 signaling pathway guarantees genome stability in the face of DNA strand breaks. Mol Cell, 51(6): 829–839
Shirra MK, McCartney R R, Zhang C, Shokat KM, Schmidt MC, Arnd K M (2008). A chemical genomics study identifies Snf1 as a repressor of GCN4 translation. J Biol Chem, 283(51): 35889–35898
Shirra M K, Rogers S E, Alexander D E, Arndt K M (2005). The Snf1 protein kinase and Sit4 protein phosphatase have opposing functions in regulating TATA-binding protein association with the Saccharomyces cerevisiae INO1 promoter. Genetics, 169(4): 1957–1972
Sies H (1982). Metabolic Compartmentation. Orlando, FL, Academic Press
Smets B, De Snijder P, Engelen K, Joossens E, Ghillebert R, Thevissen K, Marchal K, Winderickx J (2008). Genome-wide expression analysis reveals TORC1-dependent and-independent functions of Sch9. FEMS Yeast Res, 8(8): 1276–1288
Smith D L, McClure J M, Matecic M, Smith J S (2007). Calorie restriction extends the chronological lifespan of Saccharomyces cerevisiae independently of the Sirtuins. Aging Cell, 6(5): 649–662
Smith J S, Boeke J D (1997). An unusual form of transcriptional silencing in yeast ribosomal DNA. Genes Dev, 11(2): 241–254
Smith J S, Brachmann C B, Celic I, Kenna M A, Muhammad S, Starai V J, Avalos J L, Escalante-Semerena J C, Grubmeyer C, Wolberger C, Boeke J D (2000). A phylogenetically conserved NAD+-dependent protein deacetylase activity in the Sir2 protein family. Proc Natl Acad Sci USA, 97(12): 6658–6663
Soontorngun N, Larochelle M, Drouin S, Robert F, Turcotte B (2007). Regulation of gluconeogenesis in Saccharomyces cerevisiae is mediated by activator and repressor functions of Rds2. Mol Cell Biol, 27(22): 7895–7905
Sporty J, Lin S J, Kato M, Ognibene T, Stewart B, Turteltaub K, Bench G (2009). Quantitation of NAD+ biosynthesis from the salvage pathway in Saccharomyces cerevisiae. Yeast, 26(7): 363–369
Staschke K A, Dey S, Zaborske J M, Palam L R, McClintick J N, Pan T, Edenberg H J, Wek R C (2010). Integration of general amino acid control and target of rapamycin (TOR) regulatory pathways in nitrogen assimilation in yeast. J Biol Chem, 285(22): 16893–16911
Steffen K K, McCormick M A, Pham K M, MacKay V L, Delaney J R, Murakami C J, Kaeberlein M, Kennedy B K (2012). Ribosome deficiency protects against ER stress in Saccharomyces cerevisiae. Genetics, 191(1): 107–118
Strahl-Bolsinger S, Hecht A, Luo K, Grunstein M (1997). SIR2 and SIR4 interactions differ in core and extended telomeric heterochromatin in yeast. Genes Dev, 11(1): 83–93
Sturgill TW, Cohen A, Diefenbacher M, Trautwein M, Martin D E, Hall M N (2008). TOR1 and TOR2 have distinct locations in live cells. Eukaryot Cell, 7(10): 1819–1830
Sun J, Kale S P, Childress A M, Pinswasdi C, Jazwinski S M (1994). Divergent roles of RAS1 and RAS2 in yeast longevity. J Biol Chem, 269(28): 18638–18645
Sutherland C M, Hawley S A, McCartney R R, Leech A, Stark M J, Schmidt M C, Hardie D G (2003). Elm1p is one of three upstream kinases for the Saccharomyces cerevisiae SNF1 complex. Curr Biol, 13(15): 1299–1305
Sutton A, Heller R C, Landry J, Choy J S, Sirko A, Sternglanz R (2001). A novel form of transcriptional silencing by Sum1-1 requires Hst1 and the origin recognition complex. Mol Cell Biol, 21(10): 3514–3522
Swinnen E, Wanke V, Roosen J, Smets B, Dubouloz F, Pedruzzi I, Cameroni E, De Virgilio C, Winderickx J (2006). Rim15 and the crossroads of nutrient signalling pathways in Saccharomyces cerevisiae. Cell Div, 1(1): 3
Tanny J C, Kirkpatrick D S, Gerber S A, Gygi S P, Moazed D (2004). Budding yeast silencing complexes and regulation of Sir2 activity by protein-protein interactions. Mol Cell Biol, 24(16): 6931–6946
Thevelein J M, Cauwenberg L, Colombo S, Donation M, Dumortier F, Kraakman L, Lemaire K, Ma P, Nauwelaers D, Rolland F, Teunissen A, Versele M, Wera S, Winderickx J, Wera S, Winderickx J, De Winde J H, Van Dijck P (2000). Nutrient-induced signal transduction through the protein kinase A pathway and its role in the control of metabolism, stress resistance, and growth in yeast. Enzyme Microb Technol, 26(9–10): 819–825
Todisco S, Agrimi G, Castegna A, Palmieri F (2006). Identification of the mitochondrial NAD+ transporter in Saccharomyces cerevisiae. J Biol Chem, 281(3): 1524–1531
Tsang F, James C, Kato M, Myers V, Ilyas I, Tsang M, Lin S J (2015). Reduced Ssy1-Ptr3-Ssy5 (SPS) signaling extends replicative life span by enhancing NAD+ homeostasis in Saccharomyces cerevisiae. J Biol Chem, 290(20):12753–12764
Ueda Y, Oshima Y (1975). A constitutive mutation, phoT, of the repressible acid phosphatase synthesis with inability to transport inorganic phosphate in Saccharomyces cerevisiae. Mol Gen Genet, 136: 255–259
Unal E, Kinde B, Amon A (2011). Gametogenesis eliminates ageinduced cellular damage and resets life span in yeast. Science, 332 (6037). 1554–1557
Urban J, Soulard A, Huber A, Lippman S, Mukhopadhyay D, Deloche O, Wanke V, Anrather D, Ammerer G, Riezman H, Broach J R, De Virgilio C, Hall M N, Loewith R (2007). Sch9 is a major target of TORC1 in Saccharomyces cerevisiae. Mol Cell, 26(5): 663–674
van der Veer E, Nong Z, O’Neil C, Urquhart B, Freeman D, Pickering J G (2005). Pre-B-cell colony-enhancing factor regulates NAD+- dependent protein deacetylase activity and promotes vascular smooth muscle cell maturation. Circ Res, 97(1): 25–34
van Oevelen C J, van Teeffelen H A, van Werven F J, Timmers H T (2006). Snf1p-dependent Spt-Ada-Gcn5-acetyltransferase (SAGA) recruitment and chromatin remodeling activities on the HXT2 and HXT4 promoters. J Biol Chem, 281(7): 4523–4531
Veatch J R, McMurray M A, Nelson Z W, Gottschling D E (2009). Mitochondrial dysfunction leads to nuclear genome instability via an iron-sulfur cluster defect. Cell, 137(7): 1247–1258
Vickers M F, Yao S Y, Baldwin S A, Young J D, Cass C E (2000). Nucleoside transporter proteins of Saccharomyces cerevisiae. Demonstration of a transporter (FUI1) with high uridine selectivity in plasma membranes and a transporter (FUN26) with broad nucleoside selectivity in intracellular membranes. J Biol Chem, 275 (34): 25931–25938
Vlahakis A, Graef M, Nunnari J, Powers T (2014). TOR complex 2-Ypk1 signaling is an essential positive regulator of the general amino acid control response and autophagy. Proc Natl Acad Sci USA, 111 (29): 10586–10591
Vlahakis A, Powers T (2014). A role for TOR complex 2 signaling in promoting autophagy. Autophagy, 10(11): 2085–2086
Voordeckers K, Kimpe M, Haesendonckx S, Louwet W, Versele M, Thevelein J M (2011). Yeast 3-phosphoinositide-dependent protein kinase-1 (PDK1) orthologs Pkh1-3 differentially regulate phosphorylation of protein kinase A (PKA) and the protein kinase B (PKB)/ S6K ortholog Sch9. J Biol Chem, 286(25): 22017–22027
Wang C, Skinner C, Easlon E, Lin S J (2009). Deleting the 14-3-3 protein Bmh1 extends life span in Saccharomyces cerevisiae by increasing stress response. Genetics, 183(4): 1373–1384
Wang J, Jiang J C, Jazwinski S M (2010). Gene regulatory changes in yeast during life extension by nutrient limitation. Exp Gerontol, 45 (7–8): 621–631
Wanke V, Cameroni E, Uotila A, Piccolis M, Urban J, Loewith R, De Virgilio C (2008). Caffeine extends yeast lifespan by targeting TORC1. Mol Microbiol, 69(1): 277–285
Wanke V, Pedruzzi I, Cameroni E, Dubouloz F, De Virgilio C (2005). Regulation of G0 entry by the Pho80-Pho85 cyclin-CDK complex. EMBO J, 24(24): 4271–4278
Wedaman K P, Reinke A, Anderson S, Yates J 3rd, McCaffery J M, Powers T (2003). Tor kinases are in distinct membrane-associated protein complexes in Saccharomyces cerevisiae. Mol Biol Cell, 14 (3): 1204–1220
Wei M, Fabrizio P, Hu J, Ge H, Cheng C, Li L, Longo V D (2008). Life span extension by calorie restriction depends on Rim15 and transcription factors downstream of Ras/PKA, Tor, and Sch9. PLoS Genet, 4(1): e13
Weinberger M, Feng L, Paul A, Smith D L Jr, Hontz R D, Smith J S, Vujcic M, Singh K K, Huberman J A, Burhans W C (2007). DNA replication stress is a determinant of chronological lifespan in budding yeast. PLoS ONE, 2(8): e748
Weindruch W, Walford R L (1998). The retardation of aging and diseases by dietary restriction. Springfield, Illinois, USA, Charles C. Thomas
Wek R C, Jackson B M, Hinnebusch A G (1989). Juxtaposition of domains homologous to protein kinases and histidyl-tRNA synthetases in GCN2 protein suggests a mechanism for coupling GCN4 expression to amino acid availability. Proc Natl Acad Sci USA, 86 (12): 4579–4583
Wiederhold E, Gandhi T, Permentier H P, Breitling R, Poolman B, Slotboom D J (2009). The yeast vacuolar membrane proteome. Mol Cell Proteomics, 8(2): 380–392
Wilson J M, Le V Q, Zimmerman C, Marmorstein R, Pillus L (2006). Nuclear export modulates the cytoplasmic Sir2 homologue Hst2. EMBO Rep, 7(12): 1247–1251
Wogulis M, Chew E R, Donohoue P D, Wilson D K (2008). Identification of formyl kynurenine formamidase and kynurenine aminotransferase from Saccharomyces cerevisiae using crystallographic, bioinformatic and biochemical evidence. Biochemistry, 47 (6): 1608–1621
Wu Z, Liu S Q, Huang D (2013). Dietary restriction depends on nutrient composition to extend chronological lifespan in budding yeast Saccharomyces cerevisiae. PLoS ONE, 8(5): e64448
Wykoff D D, O’Shea E K (2001). Phosphate transport and sensing in Saccharomyces cerevisiae. Genetics, 159(4): 1491–1499
Xiao B, Heath R, Saiu P, Leiper F C, Leone P, Jing C, Walker P A, Haire L, Eccleston J F, Davis C T, Martin S R, Carling D, Gamblin S J (2007). Structural basis for AMP binding to mammalian AMPactivated protein kinase. Nature, 449(7161): 496–500
Xie J, Pierce M, Gailus-Durner V, Wagner M, Winter E, Vershon A K (1999). Sum1 and Hst1 repress middle sporulation-specific gene expression during mitosis in Saccharomyces cerevisiae. EMBO J, 18 (22): 6448–6454
Xu Y F, Létisse F, Absalan F, Lu W, Kuznetsova E, Brown G, Caudy A A, Yakunin A F, Broach J R, Rabinowitz J D (2013). Nucleotide degradation and ribose salvage in yeast. Mol Syst Biol, 9(1): 665
Yang J, Dungrawala H, Hua H, Manukyan A, Abraham L, Lane W, Mead H, Wright J, Schneider B L (2011). Cell size and growth rate are major determinants of replicative lifespan. Cell Cycle, 10(1): 144–155
Yao Y, Tsuchiyama S, Yang C, Bulteau A L, He C, Robison B, Tsuchiya M, Miller D, Briones V, Tar K, Potrero A, Friguet B, Kennedy B K, Schmidt M (2015). Proteasomes, Sir2, and Hxk2 form an interconnected aging network that impinges on the AMPK/Snf1-regulated transcriptional repressor Mig1. PLoS Genet, 11(1): e1004968
Young J D, Yao S Y, Sun L, Cass C E, Baldwin S A (2008). Human equilibrative nucleoside transporter (ENT) family of nucleoside and nucleobase transporter proteins. Xenobiotica, 38(7–8): 995–1021
Zaborske J M, Narasimhan J, Jiang L,Wek S A, Dittmar K A, Freimoser F, Pan T, Wek R C (2009). Genome-wide analysis of tRNA charging and activation of the eIF2 kinase Gcn2p. J Biol Chem, 284(37): 25254–25267
Zaborske JM, Wu X, Wek R C, Pan T (2010). Selective control of amino acid metabolism by the GCN2 eIF2 kinase pathway in Saccharomyces cerevisiae. BMC Biochem, 11(1): 29
Zaman S, Lippman S I, Schneper L, Slonim N, Broach J R (2009). Glucose regulates transcription in yeast through a network of signaling pathways. Mol Syst Biol, 5: 245
Zargari A, Boban M, Heessen S, Andréasson C, Thyberg J, Ljungdahl P O (2007). Inner nuclear membrane proteins Asi1, Asi2, and Asi3 function in concert to maintain the latent properties of transcription factors Stp1 and Stp2. J Biol Chem, 282(1): 594–605
Zhai R G, Zhang F, Hiesinger P R, Cao Y, Haueter C M, Bellen H J (2008). NAD synthase NMNAT acts as a chaperone to protect against neurodegeneration. Nature, 452(7189): 887–891
Zhang T, Péli-Gulli M P, Yang H, De Virgilio C, Ding J (2012). Ego3 functions as a homodimer to mediate the interaction between Gtr1-Gtr2 and Ego1 in the ego complex to activate TORC1. Structure, 20 (12): 2151–2160
Zitomer R S, Lowry C V (1992). Regulation of gene expression by oxygen in Saccharomyces cerevisiae. Microbiol Rev, 56(1): 1–11
Zuin A, Carmona M, Morales-Ivorra I, Gabrielli N, Vivancos A P, Ayté J, Hidalgo E (2010). Lifespan extension by calorie restriction relies on the Sty1 MAP kinase stress pathway. EMBO J, 29(5): 981–991
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsang, F., Lin, SJ. Less is more: Nutrient limitation induces cross-talk of nutrient sensing pathways with NAD+ homeostasis and contributes to longevity. Front. Biol. 10, 333–357 (2015). https://doi.org/10.1007/s11515-015-1367-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11515-015-1367-x