Abstract
Egg yolk, due to its emulsifying properties has a long – term tradition in food technology applications. Additionally, egg yolk extracts obtained through simple procedures were proved to be an attractive alternative to highly purified phospholipids. The aim of this work was to analyse the interfacial behaviour of previously described extracts in relation to liposomes preparation. The extracts underwent analysis of surface properties: the π-A isotherm, dilatational and stress rheology experiments as well as surface potential analysis with the use of Langmuir trough. It was proved that EA, MA and HE films were characterized by the highest collapse pressure during compression, but revealed relatively large hysteresis, suggesting irreversibility. CE extract showed minor hysteresis and high reversibility of orientation changes. The most important factor determining the elastic response on area deformation is the content of phospholipids. The lysophosphatidylcholine/phospholipids ratio is also the important factor. The balance between polar and non-polar fraction of lipids and high content of phopsholipds fraction in the film are conducive for solid-like response on shear stress whereas the presence of lysophosphatidylethanolamine induce fluid-like behaviour of this complex film. Minor film constituents significantly affect properties of the film too. MA and EA extracts revealed the highest similarity. CE and HE extracts also showed significant similarity to each other, whereas H extract differed from each.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since their discovery in 1967, by Bangham et al., liposomes have been used in membrane studies and as carriers for drugs and bioactive substances [1, 2]. For years, a few pharmaceutical products based on liposomal technology have been developed and launched. The very first one, which gained regulatory approval, Doxil® was quickly followed by Deocyt®, DuanoXome®, Mepact®, Myocyt®, Marqibo® and others [3]. Today, still new delivery systems, like ion-induced aggregated vesicles are under development [4]. Liposomes could be successfully used in food technology as well to protect bioactive compounds [5] added to health-promoting foods [1], which may cause unwanted changes in physicochemical or sensory characteristics [2, 3, 6, 7]. Those nanocapsules displaced microcapsules due to greater surface area, better solubility and minimal impact on products sensory properties. Consequently, liposomal forms of antioxidants, enzymes, antimicrobials, preservatives and undesirable flavours or odours were developed [8, 9]. Apart from industry branch, liposomes’ features define possible applications. Small unilamellar vesicles (diameter < 100 nm) are generally used as intravenous drugs, because of better distribution and long circulation time in the blood stream. Multilamellar liposomes are proper delivery systems for lipophilic drugs with affinity to lipid bilayer, while unilamellar ones – for hydrophilic compounds [2, 10]. Functionalised, multifunctional liposomes are promising especially in cancer treatment. However, their large-scale production is still under development [11]. Nevertheless, intravenous route of administration requires high purity of phospholipids and strictly determines carriers’ size as well as structure. This results in the high price of such nanocapsules. For food application, however, price is of crucial importance. Hence, there is a need to look for alternative raw materials useful for formation of liposomes for food applications. An attractive alternative for bioactive compounds encapsulation are aerogels made using biopolymers as matrix constituents. The development these two emerging technologies will probably take place independently as stabilization of bioaerogels (like emulsions) is controlled mainly by biopolymer used, whereas stabilization of liposome involve different mechanisms, among others, Zeta potential [12, 13].
The increasing interest in monolayer studies of phospholipids and other components of liposomes bilayer is observed. They are taken in addition to standard liposomes characterisation methods such as size and surface charge measurements, microscopy studies, thermodynamic properties, drug loading and release efficacy, stability etc., in order to help in understanding the mechanisms of bilayer formation. In monolayer studies, the ability of phospholipids and other surfactants to form films at the air/water interface is exploited. Properties and structure of these films depend on their composition and on interactions between surfactant molecules i.e. concentration in the interface. In pure phospholipid films molecules packaging depends mainly on the alkyl chain length [14]. However, the most important for the development of liposome technology are monolayer studies in complex systems. Special attention was paid to interaction between phospholipids and cholesterol which is often used for increasing of liposome stability bilayer permeability modulation [15, 16]. Cholesterol interacts with phospholipids molecules resulting in the increased packing densities of phospholipids molecules [17]. Moreover, monolayer studies were performed in order to look for potential alternatives to: phospholipids or cholesterol, to liposomes stabilization by freeze-drying, to improve encapsulation stability and assess interactions with encapsulated substances [18,19,20]. It should be emphasized that, phenomena taking place in both monolayer and bilayer systems are multifactorial controlled and depend not only on the film composition but also on several environmental factors as temperature or pH value [16].
Egg yolk is a natural oil-in-water emulsion. It reveals multifunctional properties, including foaming, emulsifying, and binding capability. Consequently, it is a key ingredient in many food products [9]. Its applicability depends on the ability of specific compounds, to adsorb at the oil/water interface [21]. Egg yolk is a complex system consisting of non-soluble protein aggregates (granules) in suspension of clear yellow fluid (plasma) which contains low-density lipoproteins (LDLs) and soluble proteins [11]. Lipids here are exclusively associated with lipoprotein assemblies. Therefore, in spite that, dry matter of egg yolk consists of 62.5% of lipids, and only 33.0% of proteins, both these fractions are surface active [22, 23]. A long tradition of using yolk in food technology is accompanied by number of papers describing its activity in formation and stabilisation of emulsions. At the same time, information regarding liposomes formation using egg yolk constituents is extremely rare [24,25,26]. In our previous work we stated that, egg yolk extracts obtained through simple procedures could be an attractive alternative to highly purified phospholipids for liposomes formation. Size, stability and structure of those vesicles are related to composition of extracts [27]. Herewith, we present the results of the thorough analysis of surface properties of previously described extracts in relation to liposomes preparation.
Materials and Methods
Materials
Five different extracts from hens’ egg yolk, marked as MA, EA, HE, C, and CE, were used as research material. The samples were differed by extraction procedure and, consequently, chemical composition. Both the extraction procedures and qualitative/quantitative analysis were described in details in our previous work [27]. In short, all samples were complex mixtures containing predominantly phospholipids and acyglycerols. Minor constituents i.e. tocopherols and carotenoids contents did not exceed 0.5%. MA and EA samples were obtained in several stages procedures employing ethanol, acetone, and hexane (MA) as well as methanol, chloroform, acetone and diethyl ether (EA). They stand out from other samples by the largest share of phopshoplipids in their composition. HE, C, and CE samples were obtained by one stage procedure employing hot ethanol, hexane and cold ethanol respectively. H sample contained the largest amount of acylglycerols. HE and CE samples were characterised by the most complex composition, and the CE extract contained the largest amount of minor constituents. All samples contained also cholesterol, however its concentrations in MA and EA samples were of one level of magnitude lower than in other extracts [Table 1].
The extracts were dissolved individually in chloroform of high purity (Uvasol, Merck) to obtain the solution of 1 mg/ml.
Isotherm Experiment
The monolayer was prepared with the use of Langmuir trough (KSV Nima, Finland) of the surface area 238 cm2. The apparatus was placed on a floating optical table (Standa, Lituana) in a laminar flow hood (Alpina, Poland) to ensure vibration-free and dust-free environment for the experiment. Ultrapure water (18 MΩ × cm ± 0.01 mN/m) was used to fill up the troμgh. Before the measurement, waters’ surface was cleaned with a suction pump until the surface pressure (SP) change resulted from maximum compression was <0.2 mN/m. A Hamilton syringe was used to spread all the extracts on the subphase; afterwards the sample was left for chloroform evaporation for 20 min. The surface pressure (π) was recorded by Wilhelmy platinum plate connected to the balance. The isotherm course after reduction of surface area (A) by symmetrical movement of two barriers at a constant rate of 5 mm/min. The temperature was constant and controlled by Julabo circulator throμghout the measurement. Each extract was analysed in a triplicate to ensure the reproducibility.
Surface Potential (SP)
The surface potential ΔV is related with the molecular density and dipole moment of the molecules in the monolayer at the interface according to the equation [28]:
where μn is normal component of the dipole moment, while ε and ε0 are permittivity of water and air respectively and A is molecular area. To determine surface potential [ΔV] non-destructive, non-contact vibrating capacitor method was applied. The surface potential was measured together with the isotherm, by surface potential meter (SPOT, KSV Nima, Finland), equipped with two electrodes: one located just above the water surface, and the counter electrode, immersed in the subphase. The surface potential was measured during the monolayer compression with the sensitivity at the level of ±1 mV.
Oscillatory Barrier Experiment
Oscillatory barrier technique was applied to assess the dilatational viscoelasticity of the phospholipid monolayers at the air-water interface. The monolayers were subjected to repeated compressions and expansions while deviations in surface pressure were continuously recorded. The frequencies were in the range from 0.02 to 0.1 Hz recorded in ten oscillation cycles each with 60 s interval between the measurements. The monolayers were compressed to the chosen surface pressures and, as the result of oscillations, dilatation modulus E was obtained. This quantity is composed of the real component-elastic modulus, E’ and an imaginary component, viscous modulus, E”, according to the equation [29]:
If the film is perfectly elastic, the imaginary modulus is equal to zero, while for a perfectly viscous monolayer the real part is zero.
Interfacial Stress Rheology
Interfacial stress rheometer (ISR, KSV Nima, Finland) integrated with Langmuir trough of surface area 586 cm2 was used to determine the monolayer response on shear deformation. The monolayers were compressed to a desired surface pressure between 5 and 35mN/m and an oscillatory force was applied onto a magnetic needle placed at the air/water interface. The shift of the probe was measured and recorded with a digital camera to obtain the elastic (G’) and the viscous (G”) modulus. The reported data was obtained at frequency equal to 0.1 Hz.
Statistical Analysis
Results are presented as means ± standard deviation from three replicates of each experiment. The differences between mean values were determined by analysis of variance (ANOVA). The post-hoc analysis was performed using Tukey’s test. Principal Component Analysis (PCA) was used for sample discrimination. Results in all the tests were considered significant at p < 0.05. The statistical analysis was performed using Statistica 10.0 software (StatSoft, Inc., Tulsa, OK).
Results and Discussion
Surface Pressure- Area Isotherms and Surface Potential Curves
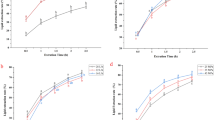
In order to determine the surface activity of the extracts, the π-A isotherms were recorded for all the samples. The obtained curves, shown in the Fig. 1, indicated several effects. The components of all extracts were able to form a surface active film at the air/water interface which is confirmed by significant growth of surface pressure during the compression. This ability is the obvious consequence of amphiphilic nature of the components of the extracts. In the region of high surface areas one can observe continuous increase of the surface pressure for all monolayers. However, at π = 15 mN/m the isotherm for H indicated sharp change of the course related to monolayer collapse. This behaviour may be explained by domination of the nonpolar fraction represented by triacylglycerols in the film formed by H extract [27]. The ratio of nonpolar/polar fraction in H is too high to form more stable film, thus the collapse occurred at relatively small surface pressure, 15 mN/m. This hypothesis is supported by the data reported by Martinet et al., [23]. By the studies of the surface activity of purified low-density lipoprotein spread at air-water interface they found that in the Π-A isotherm of neutral lipids film shows the barrier at Π = 14 mN/m [23]. The further growth of surface pressure may be related with multilayer formation. This explanation is in agreement with surface potential curve for H shown in Fig. 2d which indicated ordering of surface dipoles up to 15 mN/m followed by successive decrease of the surface potential value.
The monolayers formed by the other extracts showed similar slope of the isotherms in the condensed state what suggests similar compressibility of these films. However, we found that collapse occurred at different surface pressures - the smallest for EA (35 mN/m) and the greatest for HE (42 mN/m) what underlines some differences in the stability of these films. All the extracts can be ordered in the following sequence according to the decreasing collapse surface pressure: πcoll HE > πcoll MA > πcoll CE > πcoll EA > πcoll H.
Additional data concerning the stability of the films can be extracted from the results of hysteresis experiment accompanied by surface potential measurement as shown in Fig. 2. The course of surface pressure hysteresis is strongly affected by the composition of the extract. Despite lower film stability during the compression we found the smallest hysteresis in surface pressure isotherm for H. Slightly larger hysteresis was observed on surface pressure-area curve for CE. The same experiments for HE, EA and MA revealed large hysteresis which suggested irreversibility of the compression for these films. The observed behaviour did not reflect only the proportions between the lipids, but also may result from larger content of tocopherols (TF) in H and CE. In the recent years, Jurak et al. [30] found that α-tocopherol plays an important role in the stabilization of biological membranes depending on the acyl chain saturation degree [29]. Thus, the observed differences in the film stability may be the result of attractive interaction of tT with unsaturated acyl chains and/or repulsive interaction with saturated acyl chains. The results shown in Fig. 2 express the effect on the properties of extracts not only different amount of phospholipids, but also different ratio of saturated/unsaturated acyl chains in these molecules. Moreover, in the case of MA and EA the composition of lipid fraction containing PC, LPC, PE, LPE and SM is the most diverse among all extracts and due to this complexity, several other effects and interactions may influence the behaviour of the films. Liposomes stability, however, is strongly related to their zeta potential. In our previous paper, we prepared and characterized liposomes from the assessed extract. Hysteresis experiment showed that, the smallest hysteresis of the film observed, the highest absolute value of liposomes zeta potential [27], what underlines the strong relation between zeta potential and overall systems stability.
For the monolayers formed by HE and CE we observed the largest difference between the initial surface pressure (at the beginning of the compression) and the final value of π (after the expansion). The difference is approx. 10 mN/m. The obtained results can be associated with larger amount of carotenoids in these samples, especially zeaxanthin which may irreversibly aggregate or form dimers in the mixed films with phospholipids depending on the stereoisomer [31] These structures are stabilized by the hydrogen bonding or van der Waals forces respectively and despite low total amounts of carotenoids in the samples the monolayer properties are affected by them.
The surface potential curves shown in Fig. 2 interpreted together with surface pressure-area isotherms indicated constant growth of surface potential value up to the collapse point attributed to the ordering of molecular dipoles. It is worth noticing, that the total surface potential growth during the compression is different for the considered extracts. The largest SP was observed for CE, but the course of the curve is affected by the gaseous region on the isotherm in which the surface potential growth reflected the increase of molecular density rather than molecular arrangement. Because of that we compared the surface potential for A > Alift-off, where Alift-off means the surface area at which surface pressure rises above 0 mN/m. From this point of view, the total SP growth was found to be similar for all the films (0.07–0.08 V). These results suggested that despite different composition of the extracts the molecules showed similar tendency to change the orientation of molecular dipoles at the interface. Also, one can conclude that head groups of both phospholipids and acyloglycerols, which are main components of all samples, contributed equally to the surface potential change. The presence of cholesterol seemed to have little effect on the total surface potential change, probably because of relatively small sterol content in the samples.
After the collapse, the surface potential was stable or started to decrease (for H extract) due to reorganization and destruction of the monomolecular film. The course of surface potential curves during the expansion showed often small instabilities, moreover for EA, MA and HE the SP value after the expansion was significantly lower than at the beginning of compression. In the case of CE we observed almost the same value of SP at the beginning and at the end of compression-expansion cycle which suggest high reversibility of orientation changes in this monolayer.
Interfacial Dilatational and Shear Rheology
The interfacial rheological properties play important role in the stability of lipid films in different environments. The liposomes in food industry or in pharmacy are often exposed to stresses which cause different regions of interface to move over each other without changing the overall surface area. This kind of stress is related with shear deformation. Alternatively, the liposomes may undergo dilatational deformation which expands or contracts the surface area [32]. Most interfaces reveal a behaviour between purely elastic (solid-like) and purely viscous (fluid-like) and this response is called viscoelastic.
We studied the results of dilatational deformations obtained for the monolayers composed of all extracts. The elastic (E’) and loss (E”) moduli were plotted against surface pressure in Fig. 3 a and b respectively. The differences in the values of both moduli between considered extracts reflected their various compositions. We observed the highest E’ and E” moduli for MA and EA extracts. These results can be explained by the highest concentration of phospholipids in EA and the lowest for H. Viscoelastic response, after the dilational perturbation, is related to the interaction of the monolayer with the sub-surfaces and not due to lateral diffusion or any reorganization within the layer itself, so pure elastic branch is associated with monolayer cohesion whereas the viscoelastic one only concerns the affinities of the layer for the aqueous and organic phases [33]. The content of phospholipids was the most important factor determining the elastic response on area deformation, however the complex composition of the samples caused that both E’ and E” changed erratically with the surface pressure. This behaviour may be related with differences in miscibility in the mixed films [34]. Moreover, the E’ and E” values for π > 30 mN/m were strongly affected by the collapse of the monolayers, especially MA and EA which collapsed at surface pressures close to 37 mN/m.
Another insight into the interfacial rheology of liposomes can be given by study of the monolayer response on shear deformation. The liposomes subjected to shear stress caused by flow and mixing often undergo deformation and change of their permeability [35, 36]. On the other hand, the shear stress applied to liposomes can be the factor determining their size during self-assembly in microfluid device, which was demonstrated by Jahn et al. 2004 [37].
In order to analyse the interfacial shear rheology of the examined films, we plotted in Fig. 4 G’ and G” versus surface pressure values for the considered extracts. The most noticeable result was the domination of G’ values for the CE extract over other films. The G’ values for CE are much higher than G” for the same extract indicating strong solid-like behaviour. Both values crossed at ~30 mN/m indicating more fluid-like behaviour of the film. This observation is consistent with the run of surface potential curve, in which at π = 30 mN/m we observed distinct change of orientation of molecular dipoles in the monolayer. Inverse observation was made for EA in which G” dominated over G’ in the whole surface pressure range. The monolayers formed by other extracts showed very similar to each other, weak response for deformation stress since G’ and G” were very low and remained constant with the surface pressure increase.
It seems that both balance between polar and non-polar fraction of lipids in CE and high PE fraction in the film were conducive for solid-like response on shear stress. On the other hand, the presence of LPE in EA seemed to induce fluid-like behaviour of this complex film.
Due to the multidirectional relationship between the composition of the extracts and the physicochemical properties of the resulting films, PCA was employed to establish the relations. Both the contents of the individual compounds and the ratio between them were taken into consideration. Two principal components (factors 1 and 2) explain 82.58% of the total variance (Fig. 5). With reference to collapse surface pressure, the loading plot (Fig. 5a) showed a positive correlation with the share of polar fraction in total acylglycerols content (PF/AG) as well as the share of phosphatidylethanolamine in total phospholipids (PE/PL). At the same time a negative correlation between collapse surface pressure and the share of non-polar fraction in total acylglycerols content (NPF/AG) as well as the share of phosphatidylcholine share in total phospholipids (PC/PL) were observed.
PCA of the loadings plot (a) and score plot (b) for the dataset in Figs. 1, 3, 4. The data on the composition of extracts were derived from our earlier work [27]. Abbreviations: PL – Phospholipids; AG – Acylglycerols; Chol – Cholesterol; PC/PL – Phosphatidylcholine share in total Phospholipids; LPC/PL – Lysophosphatidylcholine share in total Phospholipids; PE/PL - Phatidylethanolamine share in total Phospholipids; LPE/PL – Lysophosphatidylethanolamine share in total Phospholipids; SM/PL – Sphingomyelin share in total Phospholipids; PF/AG - Polar fraction share in total Acylglycerols; NPF/AG – Non-polar fraction share in total Acylglycerols; α-T - -alfa-tocopherol; γ-T - gamma-tocopherol; tT – Total tocopherols; L – Lutein; Z – Zeaxanthin; C – Carotenoids; E’ – Dilatational elastic modulus; E” – Dilatational loss modulus; G’ – Shear elastic modulus; G” – Shear loss modulus; CSP - Collapse surface pressure
As regards interfacial rheology parameters it was visible that, they are determined by the presence of various components of the extract. However, the location of the points assigned to particular modulus pointed that, similar factors influenced interfacial elastic dilatational (E’) and shear viscous (G”) moduli, whereas there are no correlation between interfacial loss dilatational E” and shear elastic G’ moduli. The main factors positively affecting E’ and G” were phospholipids content (PL) and the share of lysophosphatidylcholine share in total phospholipids (LPC/PL). Negatively E’ and G” were influenced by acylglycerol (AG) and alfa-tocopherol (α-T) content as well as the share of phosphatidylcholine in total phospholipids (PC/PL). The other modules were affected mainly by the extracts components not mentioned before. So interfacial loss dilatational E” modulus was positively affected by the ratio of lysophosphatidylethanolamine share in total phospholipids (LPE/PL) and negatively by total tocopherols content (tT). Interfacial shear elastic G’ modulus was positively affected by zeaxanthin and other carotenoids content whereas negatively by the share of non-polar fraction in total acylglycerols (NPF/AG).
The score plot (Fig. 5b) allowed direct comparison of samples. It proved the high similarity of the MA and EA extracts. CE and HE extracts also showed significant similarity to each other, whereas H extract differed from each.
Conclusions
Egg yolk extracts are able to form a surface active film at the air/water interface, however, their interfacial behaviour is strongly affected by their composition. According to the collapse surface pressure H extract distinguished by high acylglycerol content was characterized by the lowest stability in monolayer. In general the ratio of polar to nonpolar fractions of acylglycerols along with the ratio of phosphatidylethanolamine to phospholipids are the most decisive factors determining the value of collapse surface pressure. Nevertheless, large hysteresis in case of films from EA, MA and HE extracts pointed at the irreversibility of the compression. At the same time, CE revealed relatively small hysteresis and high reversibility of orientation changes.
As regards interfacial rheology parameters, the most important factor determining the elastic response on area deformation is the content of phospholipids. Therefore, the highest dilatational E’ and E” moduli revealed MA and EA extracts. The lysophosphatidylcholine/ phospholipids ratio is also an important factor. In reference to interfacial shear rheology the balance between polar and non-polar fraction of lipids and high content of phopsholipds fraction in the film are conducive for solid-like response on shear stress. On the other hand, the presence of lysophosphatidylethanolamine induced fluid-like behaviour of this complex film.
Minor film constituents significantly affected properties of film too. Tocopherols negatively influenced both interfacial shear moduli and dilatational elastic E’ modulus. The presence of carotenoids positively affected interfacial shear elastic G’.
In terms of interfacial behaviour, MA and EA extracts revealed the highest similarity. CE and HE extracts were significantly alike as well, whereas H extract differed from each.
Abbreviations
- LDL:
-
Low-density lipoprotein
- EA:
-
Ethanol/acetone extract
- MA:
-
Methanol-chloroform/acetone extract
- HE:
-
Hot ethanol extract
- H:
-
Hexane extract
- CE:
-
Cold ethanol extract
- ANOVA:
-
Analysis of variance
- PCA:
-
Principal component analysis
- Π:
-
Surface pressure [mN/m]
- A:
-
Area [cm2]
- SP:
-
Surface potential [ΔV]
- CSP:
-
Collapse Surface Pressure
- Z:
-
Zeaxanthin
- C:
-
Carotenoids
- L:
-
Lutein
- Chol:
-
Cholesterol
- tT:
-
Total tocopherols
- α-T:
-
alfa-tocopherol
- γ-T:
-
gamma-tocopherol
- AG:
-
Acylglycerols
- PL:
-
Phospholipids
- PC:
-
Phosphatidylcholine
- LPE:
-
Lysophosphatidylethanolamine
- LPC:
-
Lysophosphatidylcholine
- SM:
-
Sphingomyelin
- NPF:
-
Non-polar fraction
- PC/PL:
-
Phosphatidylcholine share in total Phospholipids
- NPF/AG:
-
Non-polar fraction share in total Acylglycerols
- LPE/PL:
-
Lysophosphatidylethanolamine share in total Phospholipids
- PE/PL:
-
Phatidylethanolamine share in total Phospholipids
- E’:
-
Dilatational elastic modulus
- E”:
-
Dilatational loss modulus
- G’:
-
Shear elastic modulus
- G”:
-
Shear loss modulus
- LPC/PL:
-
Lysophosphatidylcholine share in total Phospholipids
- SM/PL:
-
Sphingomyelin share in total Phospholipids
- PF/AG:
-
Polar fraction share in total Acylglycerols
References
A.D. Bangham, M.M. Standish, G. Wessmann, J. Mol. Biol. (1965). https://doi.org/10.1016/S0022-2836(65)80094-8
A. Akbarzadeh, R. Razaei-Sadabady, S. Davaran, W.S. Joo, N. Zarghami, Nanoscale Res. Lett. (2013). https://doi.org/10.1186/1556-276X-8-102
U. Bulbake, S. Doppolapudi, N. Kommineni, W. Khan, Pharmaceutics (2017). https://doi.org/10.3390/pharmaceutics9020012
R. Ran, A. Middelberg, C.-X. Zhao, Colloid Surface B (2016). https://doi.org/10.1016/j.colsurfb.2016.09.016
M. Kujawska, A. Olejnik, G. Lewandowicz, P. Kowalczewski, R. Forjasz, J. Jodynis-Liebert, Nutrients (2018). https://doi.org/10.3390/nu10020259
P. Kowalczewski, M. Różańska, A. Makowska, P. Jeżowski, P. Kubiak, Food Sci. Technol. Int. (2018). https://doi.org/10.1177/1082013218814605
M.H. Baranowska, Ł. Masewicz, P.Ł. Kowalczewski, G. Lewandowicz, M. Piątek, P. Kubiak, Eur. Food Res. Technol. (2018). https://doi.org/10.1007/s00217-017-2965-4
M. Fathi, M.R. Mozafari, M. Mohebbi, Trends Food Sci. Technol. (2012). https://doi.org/10.1016/j.tifs.2011.08.003
P. De Silva Malheiros, D.J. Daroit, A. Brandelli, Trends Food Sci. Technol. (2010). https://doi.org/10.1016/j.tifs.2010.03.003
J.O. Eloy, M.C. Souza, R. Petrilli, J.P.A. Barcellos, R.J. Lee, M.J. Machetti, Colloid Surface B (2014). https://doi.org/10.1016/j.colsurfb.2014.09.029
M. Anton, J. Sci. Food Agric. (2013). https://doi.org/10.1002/jsfa.6247
K. Gansen, T. Budtova, L. Ratke, P. Gurikov, V. Baudron, I. Preibisch, P. Niemieyer, I. Smirnova, B. Milow, Materials (2018). https://doi.org/10.3390/ma11112144
C. Yan, P.S. Given, G. Huvard, R.R. Mallepally, M.A. McHugh, Method of loading flavour into aerogel and flavour based on food grade materials. United States Patent, March, 2016 [US 2016/0058045 A1]
B. Moghaddam, M.H. Ali, J. Wilkhu, D.J. Kirby, A.F. Mohammed, Q. Zheng, Y. Perrie, Int. J. Pharm. (2011). https://doi.org/10.1016/j.ijpharm.2011.01.020
P. Dynarowicz-Latka, K. Hac-Wydro, Colloid Surface B (2004). https://doi.org/10.1016/j.colsurfb.2004.06.007
W.W. Sulkowski, D. Pentak, K. Nowak, A. Sulkowska, J. Mol. Struct. (2005). https://doi.org/10.1016/j.molstruc.2004.11.075
M. Doxastakis, A.K. Sum, J.J. de Pablo, J. Phys. Chem. B (2005). https://doi.org/10.1021/jp054843u
M.H. Ali, D.J. Kirby, A.R. Mohammed, Y. Perrie, J. Pharm. Pharmacol. (2010). https://doi.org/10.1111/j.2042-7158.2010.01090.x
G. Brezesinski, H. Mohwald, Adv. Colloid Interf. Sci. (2003). https://doi.org/10.1016/s0001-8686(02)0071-4
D. Christensen, D. Kirby, C. Foged, E.M. Agger, P. Andersen, Y. Perrie, H.M. Nielsen, BBA – Biomembranes (2008). https://doi.org/10.1016/j.bbamem.2008.01.013
H. Motta-Romero, Z. Zhang, A.-T. Nguyen, V. Schlegel, Y. Zhang, J. Food Sci. (2017). https://doi.org/10.1111/1750-3841.13747
R. Huopalathi, R. López-Fandiño, M. Anton, R. Schade, Bioactive Egg Compounds (Springer-Verlag, Berlin Heidelberg, 2007), pp. 1–6
V. Martinet, P. Saulnier, V. Beaumal, J.L. Courthaudon, M. Anton, Colloid Surface B (2003). https://doi.org/10.1016/S0927-7765(03)00139-5
R. Wałęsa, D. Man, G. Engel, D. Siodłak, T. Kupka, T. Ptak, M. Broda, Chem. Biodivers. (2015). https://doi.org/10.1002/cbdv.201400179
A. Paraskevopoulou, V. Kiosseoglou, S. Pegiadou, J. Agric. Food Chem. (1997). https://doi.org/10.1021/jf9701188
A. Bryła, G. Lewandowicz, W. Juzwa, J. Food Eng. (2015). https://doi.org/10.1016/j.jfoodeng.2015.07.025
A. Kondratowicz, G. Neunert, N. Niezgoda, J. Brys, A. Siger, M. Rudzinska, G. Lewandowicz, J. Food Sci. (2018). https://doi.org/10.1111/1750-3841.14341
D.M. Taylor, G.F. Bayes, Mat Sci Eng C (1999). https://doi.org/10.1016/S0928-4931(99)00064-8
R. Miller, L. Liggieri, Interfacial Rheology, 1st edn. (CRC Press Book, 2009), pp. 1–24
M. Jurak, C.J. Miñones, BBA – Biomembranes (2013). https://doi.org/10.1016/j.bbamem.2013.07.005
J. Milanowska, A. Polit, Z. Wasylewski, W. Gruszecki, J Photochem Photobiol (2003). https://doi.org/10.1016/j.jphotobiol.2003.08.009
D.J. McClements, Food Emulsions: Principles, Practice, and Techniques, 3rd edn. (CRC Press Book 2004), pp. 184–244
N. Anton, P. Saulnier, F. Boury, F. Foussard, J.-P. Benoit, J.E. Proust, Chem. Phys. Lipids (2007). https://doi.org/10.1016/j.chemphyslip.2007.06.226
S. Dauphas, V. Beaumal, P. Gunning, A. Mackie, P. Wilde, V. Vié, A. Riaublanc, M. Anton, Colloid Surface B (2007). https://doi.org/10.1016/j.colsurfb.2007.01.017
S.R. Chakravarthy, T.D. Giorgio, BBA – Biomembranes (1992). https://doi.org/10.1016/0005-2736(92)90392-Y
A.L. Bernard, M.A. Guedeau-Boudeville, V. Marchi-Artzner, T. Gulik-Krzywicki, J.M. Meglio, L. Jullien, J. Colloid Interface Sci. (2005). https://doi.org/10.1016/j.jcis.2004.12.019
A. Jahn, W.N. Vreeland, M. Gaitan, L.E. Locascio, J. Am. Chem. Soc. (2004). https://doi.org/10.1021/ja0318030
Acknowledgements
The work was financially supported by the funds of Department of Biotechnology and Food Microbiology, Poznan University of Life Sciences within the project No 508.771.01. The author K.D. wishes to acknowledge Polish Ministry of Science and Higher Education for the financial support (Grant. No. 03/32/DSPB/0801).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
OpenAccess This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kondratowicz, A., Dopierała, K. & Lewandowicz, G. Interfacial Behaviour of Egg Yolk Extracts. Food Biophysics 14, 205–213 (2019). https://doi.org/10.1007/s11483-019-09572-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11483-019-09572-4