Abstract
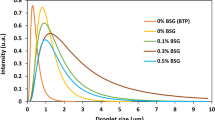
This study aimed to evaluate the ability of commercial soy protein isolate (SPI) to form cold-set gels under different pHs (5–11), pre-heating temperatures (60 °C, 80 °C), CaCl2 (0–15 mM) and SPI (5–15%, w/v) concentrations, and also select a formulation for the investigation of the effects of incorporating locust bean gum (LBG) (0–0.3%, w/v) and solid lipid microparticles (SLM) on gels rheological and microstructural properties. Gels were evaluated in terms of visual aspect, water-holding capacity, microstructure (using confocal laser scanning microscopy and cryo-scanning electronic microscopy) and rheological properties. SPI showed higher solubilities at pHs 7 (32.0%), 9 (51.6%) and 11 (100%). Self-supported gels were obtained under several conditions at alkaline pHs. At pH 7, only systems pre-heated to 80 °C with 15% (w/v) SPI and 10 or 15 mM CaCl2 gave self-supported gels. At neutral pH, samples showed relative structural instability, which was minimized with LBG incorporation. Formulations GSPI (pH 7, preheated to 80 °C, 15% (w/v) SPI, 10 mM CaCl2) and GMIX (pH 7, preheated to 80 °C, 15% (w/v) SPI, 0.2% (w/v) LBG, 15 mM CaCl2) were selected for emulsion-filled gels (EFG) production. Power law parameters (K′, K″), calculated from frequency sweep results, revealed that non-filled GMIX (K′: 472.1; K″: 77.6) was stronger than GSPI (K′: 170.4; K″: 33.6). Besides, GMIX showed microphase separation. SLM stabilized with Tween 80-Span 80 were active fillers in EFG, altering microstructures and increasing G’, G” and the Young’s modulus (1.8 to 2.1 kPa for GSPI and 1.4 to 2.2 kPa for GMIX).




Similar content being viewed by others
References
K. Nishinari, Y. Fang, S. Guo, G.O. Phillips, Food Hydrocoll. 39, 301–318 (2014)
A. Maltais, G.E. Remondetto, M. Subirade, Food Hydrocoll. 22(4), 550–559 (2008)
J.A.P. Vilela, A.L.F. Cavallieri, R.L. Cunha, Food Hydrocoll. 25(7), 1710–1718 (2011)
A.C. Alting, H.H.J. de Jongh, R.W. Visschers, J.F.A. Simons, J. Agric. Food Chem. 50(16), 4682–4689 (2002)
C. Rocha, J.A. Teixeira, L. Hilliou, P. Sampaio, M.P. Gonçalves, Food Hydrocoll. 23(7), 1734–1745 (2009)
A.L.F. Cavallieri, R.L. Cunha, Food Biophys. 4(2), 94–105 (2009)
A.C. Alting, R.J. Hamer, C.G. de Kruif, M. Paques, R.W. Visschers, Food Hydrocoll. 17(4), 469–479 (2003)
Y. Chang, D. Li, L. Wang, C. Bi, B. Adhikari, Carbohydr. Polym. 108, 183–191 (2014)
M.C. Puppo, D.A. Sorgentini, M.C. Añón, J. Am. Oil Chem. Soc 80(6), 605–611 (2003)
C. Tang, X. Wang, X. Yang, L. Li, J. Food. Eng 92, 432–437 (2009)
K.R. Kuhn, A.L.F. Cavallieri, R.L. Cunha, Food Hydrocoll. 25(5), 1302–1310 (2011)
M. Bertrand, S.L. Turgeon, Food Hydrocoll. 21(2), 159–166 (2007)
V.J. Morris, Curr. Opin. Colloid Interface Sci. 2(6), 567–572 (1997)
S. Jong, F. Van De Velde, Food Hydrocoll. 21(7), 1172–1187 (2007)
S. Jong, H. Jan Klok, F. Van De Velde, Food Hydrocoll. 23(3), 755–764 (2009)
S.R. Monteiro, S. Rebelo, O.A.B.C. Silva, J.A. Lopes-da-Silva, Food Hydrocoll. 33(2), 349–360 (2013)
C. Bi, D. Li, L. Wang, B. Adhikari, LWT - Food Sci. Technol. 75, 1–8 (2007)
S. Barak, D. Mudgil, Int. J. Biol. Macromol. 66, 74–80 (2014)
D.E. Dunstan, Y. Chen, M.-L. Liao, R. Salvatore, D.V. Boger, M. Prica, Food Hydrocoll. 15(4–6), 475–484 (2001)
C. Sandolo, D. Bulone, M.R. Mangione, S. Margheritelli, C.D. Meo, F. Alhaique, P. Matricardi, T. Coviello, Carbohydr. Polym. 82(3), 733–741 (2010)
G. Lorenzo, N. Zaritzky, A. Califano, Food Hydrocoll. 30(2), 672–680 (2013)
K. Liu, M. Stieger, E. van der Linden, F. van de Velde, Food Hydrocoll. 44, 244–259 (2015)
L. Oliver, E. Scholten, G.A. van Aken, Food Hydrocoll. 43, 299–310 (2015)
G. Sala, G.A. van Aken, M.A.C. Stuart, F. van de Velde, J. Texture Stud. 38(4), 511–535 (2007)
G. Sala, T. van Vliet, M.A.C. Stuart, G.A. van Aken, F. van de Velde, Food Hydrocoll. 23, 1853–1863 (2009)
G. Sala, T. van Vliet, M.A.C. Stuart, G.A. van Aken, F. van de Velde, Food Hydrocoll. 23(5), 1381–1393 (2009)
R. Davies, D.E. Graham, B. Vincent, J. Colloid Interface Sci. 116(1), 88–89 (1987)
C.V. Morr, B. German, J.E. Kinsella, J.M. Regenstein, J.P. Van Buren, A. Kilara, B.A. Lewis, M.E. Mangino, J. Food Sci. 50(6), 1715–1718 (1985)
T.C. Brito-Oliveira, M. Bispo, I.C.F. Moraes, O.H. Campanella, S.C. Pinho, Food Res. Int. 102, 759–767 (2017)
F.A. Perrechil, A.C.K. Sato, R.L. Cunha, J. Food Eng. 104(1), 123–133 (2011)
B.C. Beuschel, J.D. Culbertson, P.A. Partridge, D.M. Smith, J. Food Sci. 57(3), 605–609 (1992)
M.J. Spotti, O. Tarhan, S. Schaffter, C. Corvalan, O.H. Campanella, Food Hydrocoll. 63, 696–704 (2016)
A.R. Abhyankar, D.M. Mulvihill, M.A.E. Auty, Food Struct. 1(2), 127–136 (2014)
N. Özkan, H. Xin, X.D. Chen, J. Food Sci. 67(5), 1814–1820 (2002)
K.H. Lee, H.S. Ryu, K.C. Rhee, JAOCS 80(1), 85–90 (2003)
J. Jiang, J. Chen, Y.L. Xiong, J. Agric. Food Chem. 57(16), 7576–7583 (2009)
A.L.M. Braga, A. Azevedo, M.J. Marques, M. Menossi, R.L. Cunha, Food Hydrocoll. 20(8), 1178–1189 (2006)
C. Wang, S. Damodaran, J. Agric. Food Chem. 39(3), 433–438 (1991)
V.E. Sánchez, G.B. Bartholomai, A.M.R. Pilosof, LWT - Food Sci. Technol. 28(4), 380–385 (1995)
P.J. Fleming, G.D. Rose, Protein Sci. 14(7), 1911–1917 (2005)
F. Ma, C. Chen, G. Sun, W. Wang, H. Fang, Z. Han, Innov. Food Sci. Emerg. Technol. 14, 31–37 (2012)
M. Yang, F. Liu, C. Tang, Food Res. Int. 52(1), 409–418 (2013)
S.L. Turgeon, M. Beaulieu, C. Schmitt, C. Sanchez, Curr. Opin. Colloid Interface Sci. 8(4-5), 401–414 (2003)
E. Dickinson, S.T. Hong, J. Agric. Food Chem. 43(10), 2560–2566 (1995)
J. Chen, E. Dickinson, M. Langton, A.M. Hermansson, LWT - Food Sci. Technol. 33(4), 299–307 (2000)
Y.L. Xiong, J.E. Kinsella, Milchwissenschaft 46(4), 207–212 (1991)
Acknowledgments
The authors thank the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for fellowships for Thais C. Brito-Oliveira (grants 2014/26106-2 and 2016/03271-3) and the University of São Paulo for a fellowship for Marina Bispo. The authors also thank Agropalma, Danisco and Cargill for donating the palm stearin, xanthan gum, and locust bean gum, respectively, and the National Institute of Science and Technology on Photonics Applied to Cell Biology (INFABiC) at the State University of Campinas (Unicamp) for access to the LSM 780 NLO-Zeiss inverted microscope (Zeiss, Germany). The authors also would like to thank Dr. Robert L Seiler of the Life Science Microscopy Facility at Purdue University for his technical assistance in cryo-SEM analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 847 kb)
Rights and permissions
About this article
Cite this article
Brito-Oliveira, T.C., Bispo, M., Moraes, I.C.F. et al. Cold-Set Gelation of Commercial Soy Protein Isolate: Effects of the Incorporation of Locust Bean Gum and Solid Lipid Microparticles on the Properties of Gels. Food Biophysics 13, 226–239 (2018). https://doi.org/10.1007/s11483-018-9529-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11483-018-9529-4