Abstract
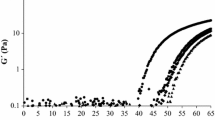
Cold-set whey protein (WP) gels with addition of xanthan or guar were evaluated by mechanical properties and scanning electron microscopy. Gels were formed after the addition of different amounts of glucono-δ-lactone to thermally denatured WP solutions, leading to different acidification rates and final pH values. At lower acidification rates and higher final pH, gels showed more discontinuous structure and weaker and less elastic network, which was attributed to a predominance of phase separation during gel formation due to slower gelation kinetics. In contrast, at higher acidification rates and lower final pHs, gelation prevailed over phase separation, favoring the formation of less porous structures, resulting in stronger and more elastic gels. The gels’ fractal dimension (D f; structure complexity) and lacunarity were also influenced by the simultaneous effects of gelation and phase separation. For systems where phase separation was the prevailing mechanism, greater lacunarity parameters were usually observed, describing the heterogeneity of pore distribution, while the opposite occurred at prevailing gelation conditions. Increase in guar concentration or lower final pH of xanthan gels entailed in D f reduction, while the increase in xanthan concentration resulted in higher D f. Such a result suggests that the network contour length was rugged, but this pattern was reduced by the increase of electrostatic interactions among WP and xanthan. Guar addition caused the formation of gel network with smoother surfaces, which could be attributed to the guar–protein excluded volume effects leading to an increase in protein–protein interactions.










Similar content being viewed by others
References
A.H. Clark, G.M. Kavanagh, S.B. Ross-Murphy, Food Hydrocoll. 15, 383–400 (2001). doi:10.1016/S0268-005X(01)00042-X
C.M. Bryant, D.J. McClements, Trends Food Sci. Technol. 9, 143–151 (1998). doi:10.1016/S0924-2244(98)00031-4
Z.Y. Ju, A. Kilara, J. Agric. Food Chem. 46, 1830–1835 (1998). doi:10.1021/jf9710185
A.C. Alting, H.H.J. de Jongh, R.W. Visschers, J. Simons, J. Agric. Food Chem. 50, 4682–4689 (2002). doi:10.1021/jf011657m
A.C. Alting, R.J. Hamer, C.G. De Kruif, M. Paques, R.W. Visschers, Food Hydrocoll. 17, 469–479 (2003). doi:10.1016/S0268-005X(03)00023-7
A.C. Alting, R.J. Hamer, C.G. De Kruif, R.W. Visschers, J. Agric. Food Chem. 51, 3150–3156 (2003). doi:10.1021/jf0209342
A. Syrbe, W.J. Bauer, N. Klostermeyer, Int. Dairy J. 8, 179–193 (1998). doi:10.1016/S0958-6946(98)00041-7
S. de Jong, H. Jan Klok, F. van de Velde, Food Hydrocoll. 23, 755–764 (2009). doi:10.1016/j.foodhyd.2008.03.017
S. de Jong, F. van de Velde, Food Hydrocoll. 21, 1172–1187 (2007). doi:10.1016/j.foodhyd.2006.09.004
A.L.F. Cavallieri, R.L. Da Cunha, Food Hydrocoll. 22, 439–448 (2008). doi:10.1016/j.foodhyd.2007.01.001
A.C. Alting, R.J. Hamer, G.G. de Kruif, R.W. Visschers, J. Agric. Food Chem. 48, 5001–5007 (2000). doi:10.1021/jf000474h
A.L.F. Cavallieri, A.P. Costa-Netto, M. Menossi, R.L. Da Cunha, Lait. 87, 535–554 (2007). doi:10.1051/lait:2007032
W.C. Wielinga, in Galactomannans, ed. by G.O. Phillips, P.A. Williams. Handbook of Hydrocolloids (CRC, Boca Raton, 2000), pp. 153–171
G. Sworn, in Xanthan gum, ed. by G.O. Phillips, P.A. Williams. Handbook of Hydrocolloids (CRC, Boca Raton, 2000), pp. 179–193
U.K. Laemmli, Nature 227, 680–685 (1970). doi:10.1038/227680a0
J.R. Hodge, B.T. Hofreiter, in Determination of reducing sugars and carbohydrates: Phenol Sulfuric test, ed. by R.L. Wistler, M.L. Wolfman. Methods in Carbohydrate Chemistry (Academic, New York, 1962), pp. 380–394
AOAC., Official Methods of Analysis of AOAC international. Association of Official Analytical Chemists (Patricia Cunniff, Gaithersburg, USA, 1997)
J.F. Steffe, Rheological Methods in Food Process Engineering (Freeman, East Lansing, USA, 1996)
L.A. Pugnaloni, L. Matia-Merino, E. Dickinson, Colloid. Surf. B. 42, 211–217 (2005). doi:10.1016/j.colsurfb.2005.03.002
B.H. Kaye, A Random Walk Through Fractal Dimensions (VHC Verlagsgesellschft, Weinheim, Germany, 1989)
E. Davila, M. Toldra, E. Saguer, C. Carretero, D. Pares, LWT-Food Sci. Technol. 40, 1321–1329 (2007)
E. Dàvila, D. Perés, Food Hydrocoll. 21, 147–153 (2007). doi:10.1016/j.foodhyd.2006.02.004
C.M. Bryant, D.J. McClements, Food Hydrocoll. 14, 383–390 (2000). doi:10.1016/S0268-005X(00)00018-7
D.V. Zasypkin, E.E. Braudo, V.B. Tolstoguzov, Food Hydrocoll. 11, 159–170 (1997)
V. Tolstoguzov, Food Hydrocoll. 17, 1–23 (2003). doi:10.1016/S0268-005X(01)00111-4
C. Sanchez, R. Zuniga-Lopez, C. Schmitt, S. Despond, J. Hardy, Int. Dairy J. 10, 199–212 (2000). doi:10.1016/S0958-6946(00)00030-3
M. Langton, A.M. Hermansson, Food Hydrocoll. 10, 179–191 (1996)
T. Hagiwara, H. Kumagai, K. Nakamura, Food Hydrocoll. 12, 29–36 (1998). doi:10.1016/S0268-005X(98)00043-5
T. Hagiwara, H. Kumagai, T. Matsunaga, J. Agric. Food Chem. 45, 3807–3812 (1997). doi:10.1021/jf970348m
A.G. Marangoni, S. Barbut, S.E. McGauley, M. Marcone, S.S. Narine, Food Hydrocoll. 14, 61–74 (2000). doi:10.1016/S0268-005X(99)00046-6
M. Mellema, J.H.J. van Opheusden, T. van Vliet, J. Rheol. (NYNY). 46, 11–29 (2002). doi:10.1122/1.1423311
M. Mellema, P. Walstra, J.H.J. van Opheusden, T. van Vliet, Adv. Colloid. Interface Sci. 98, 25–50 (2002). doi:10.1016/S0001-8686(01)00089-6
Q.X. Zhong, C.R. Daubert, O.D. Velev, Langmuir. 20, 7399–7405 (2004). doi:10.1021/la036147w
Q.X. Zhong, C.R. Daubert, O.D. Velev, J. Agric. Food Chem. 55, 2688–2697 (2007). doi:10.1021/jf0625914
A. Barret, M. Peleg, Food Sci. Technol.-Lebensm.–Wiss. Technol. 28, 553–563 (1995). doi:10.1016/0023-6438(95)90001-2
Acknowledgments
This research was supported by FAPESP (04/08517-8) and CNPq (301869/2006-5 and 140506/2003-9).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cavallieri, Â.L.F., Cunha, R.L. Cold-Set Whey Protein Gels with Addition of Polysaccharides. Food Biophysics 4, 94–105 (2009). https://doi.org/10.1007/s11483-009-9105-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11483-009-9105-z