Abstract
Cerebral ischemia reperfusion (I/R) is one of the neurovascular diseases which leads to severe brain deterioration. Haemorrhagic transformation (HT) is the main complication of ischemic stroke. It exacerbates by reperfusion, causing a more deleterious effect on the brain and death. The current study explored the protective effect of sertraline (Sert) against cerebral I/R in rats by inhibiting HT, together with the molecular pathways involved in this effect. Forty-eight wister male rats were divided into 4 groups: Sham, Sert + Sham, I/R, and Sert + I/R. The ischemic model was induced by bilateral occlusion of the common carotid artery for 20 min, then reperfusion for 24 h. Sertraline (20 mg/kg, p.o.) was administrated for 14 days before exposure to ischemia. Pre-treatment with Sert led to a significant attenuation of oxidative stress and inflammation. In addition, Sert attenuated phosphorylation of extracellular regulated kinases and nuclear factor kappa—p65 expression, consequently modulating microglial polarisation to M2 phenotype. Moreover, Sert prevented the hemorrhagic transformation of ischemic stroke as indicated by the notable decrease in neuronal expression of CD163, activity of Heme oxygenase-2 and matrix metalloproteinase-2 and 9 levels. In the same context, Sert decreased levels of autophagy and apoptotic markers. Furthermore, histological examination, Toluidine blue, and Prussian blue stain aligned with the results. In conclusion, Sert protected against cerebral I/R damage by attenuating oxidative stress, inflammation, autophagy, and apoptotic process. It is worth mentioning that our study was the first to show that Sert inhibited hemorrhagic transformation.
Graphical Abstract
The protective effect of sertraline against injury induced by cerebral ischemia reperfusion via inhibiting Hemorrhagic transformation.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ischemia occurs due to obstruction of the blood vessels and leads to a decrease in oxygen and glucose required for cellular demands, thus causing tissue injury. On the other hand, reperfusion restores the blood flow, an event which leads to a more aggressive deterioration to the tissue. Cerebral I/R injury takes place after partial restoration of blood flow (Wanchao et al. 2018; Wang et al. 2020). Moreover, it is significant to mention that ischemic stroke is one of the detrimental causes of disability worldwide (Wu et al. 2017).
Restoration of blood flow to brain tissue after ischemia results in a tremendous reactive oxygen species (ROS) production, which causes upregulated expression of inflammatory mediators (Jurcau and simion 2022). This inflammatory process happens at 3 levels as follows: acute stage (the first hours of incidence of stroke), in which microglia damage the degenerated cells and leukocytes infiltrate, commonly neutrophils; the subacute stage, which relies upon the release of the inflammatory mediators; late-stage depends on astrocyte and microglial cell reparatory role (Jurcau and simion 2022).Consequently, due to the inflammatory status of the brain tissue, the apoptotic cascade is activated either directly or indirectly. Finally, these destructive manifestations disrupt the blood membrane barrier, edema, and hemorrhagic transformation (HT) (Jurcau and simion 2022).
Microglial cells are native immune cells in the central nervous system which check the brain parenchyma. According to the type of stimulus, microglial cells are activated and switched between two polarisation phenotypes, the phenotype (M1) and the alternative phenotype (M2) (Zhang et al. 2019; Yu et al. 2020). Microglial cells can take an inflammatory or pro-inflammatory role via switching between M1 and M2-activated phenotypes, affecting the progression of ischemic stroke. The microglial cell shifts to the M1 phase during cerebral I/R, due to the release of inflammatory mediators, ROS, DAMPs, and neuromodulators from degenerated neurons in the ischemic core and activation of nuclear factor kappa-β (Fernandez-Lizarbe et al. 2009). Cytotoxic factors such as inflammatory mediators and nitric oxide are released from the M1 phenotype, which also disrupts the blood–brain barrier (BBB), increases its permeability, and engulfs vascular endothelial cells, causing a breakdown of this barrier after stroke (Xu et al. 2020).On the other hand, M2 polarisation enhances anti-inflammatory factors, eliminates debris, induces angiogenesis, and repairs peripheral injured neurons (Dong et al. 2021). Also, the M1 phenotype has a role in amplifying necroptosis of endothelial cells and disruption of BBB following ischemic stroke (Jiang et al. 2020). All these together cause HT that is detected by numerous markers such as CD 163, which acts as a scavenger receptor on neurons for haemoglobin (Hb), allowing safe getting rid of Hb without toxic effects that worsen brain status after stroke (Leclerc et al. 2018).
Autophagy is a fundamental biological process that causes the breakdown and reprocessing of cellular constituents and consequently underlies many physiological and pathological conditions such as diabetes, neurodegenerative diseases, and obesity. It is reported that autophagy participates in ischemic diseases such as myocardial infarction, focal ischemia, global ischemia, cerebral I/R injury, and hypoxic ischemia (Sun et al. 2020; Zhang et al. 2020).
Selective serotonin reuptake inhibitors (SSRI) are resorted to for managing many psychological diseases, mainly anxiety disorders. Sertraline (Sert) is one of the most prescribed SSRI drugs for managing several conditions, such as anxiety, depression, and obsessive–compulsive disease (Atli et al. 2017). Previous studies documented the anti-inflammatory effect of Sert through decreasing production of cyclooxygenase-2, TNF-α and IL-1β (Baharav et al. 2012; Sitges et al. 2014). Also, Sert exhibited antioxidant, antiapoptotic and neurogenic effects (Ozturk et al. 2013).
It is noteworthy that only a few studies have investigated hemorrhagic transformation as a secondary impact of cerebral stroke on brain injury and how it increases the rate of morbidity and mortality. Hence, our study was established aiming to assess the protective potential of Sert against cerebral I/R-induced brain injury in rats. In parallel, the study focused on investigating the molecular pathways impacted by Sert, specifically microglial polarisation and autophagy, along with the relevance of these molecular pathways to the inhibition of the transformation of ischemic stroke to hemorrhagic stroke.
Materials and Methods
Drugs
Sert was kindly granted by the Egyptian Company for Chemicals and Pharmaceuticals, Cairo, Egypt. All solvents and chemicals were of analytical grade.
Animals
Forty-eight Wister male rats weighing 180 to 200 gm were purchased from the breeding unit of the Egyptian Organization of Biological Products and Vaccines. Approval of the experimental protocol was taken from the ethical committee at the Faculty of Pharmacy, Helwan University (Protocol Number: 05A2021). Randomly, the rats were distributed (4 rats per cage) at a controlled temperature (22 ± 2 0c) and environmental settings. A week before the experiment, they were habituated with free access to a standard pellet diet and drinking water ad libitum.
Experimental Groups and Treatment
Randomly, animals were distributed into 4 groups (12rats/group). The first group (Sham group) and the second group (Sert + Sham group) were exposed to the identical surgical steps of cerebral I/R injury without occlusion of the carotid artery and received saline (1 ml/kg; p.o.) and Sert (20 mg/kg; p.o.) respectively, for 14 days. The third group (I/R group) and the fourth group (Sert + I/R group) received saline (1 ml/kg; p.o.) and Sert (20 mg/kg; p.o.) respectively, for 14 days (Shin et al. 2009) followed by cerebral I/R injury. Following cerebral I/R injury, the rats were decapitated, and the brain was excised to be utilised for further studies.
Induction of Cerebral I/R
Twenty-four rats were randomly chosen, fasted overnight, and anesthetised using chloral hydrate (360 mg/kg, i.p) at the time of surgery. For induction of ischemia, the carotid arteries were bilaterally ligated for 20 min. Afterwards, the circulation was restored through the de-ligation of arteries, and reperfusion was allowed for 24 h (Jin et al. 2019).
After 24 h reperfusion, rats were decapitated; brains were immediately isolated from the skull on ice, and rinsed with ice-cold phosphate-buffered saline (PBS). The samples were distributed as follows:
The left hemisphere (6 per group) was homogenised in PBS to get 10% W/V homogenate, then they were stored at -20 0C. Homogenates were exposed to two freeze–thaw cycles to disturb the cell membrane. After that, the homogenate was centrifuged at 40 C for 5 min at 5000 rpm, then the supernatants were set for biochemical analysis, while the right hemisphere (6 per group) was set for western blot measures.
The other six brains from each group were fixed for histopathological investigation.
Biochemical Assay
Glutathione (GSH), nitrate/nitrite and malondialdehyde (MDA) levels were estimated in whole brain tissue using kits; Bio-Vision (Milpitas, CA, USA), Cayman chemical (Ann Arbor, MI, USA) and Sigma-Aldrich Co. (St. Louis, MO, USA) respectively. Furthermore, ELISA kits were utilised for the estimation of BcL2, Bax, TNF‐α, matrix metalloproteinase 2,9 (MMP-2,9) and IL‐10 levels (CUSABIO, Wuhan, China), IL-1β (MyBioSource, San Diego, CA, USA), Heme oxygenase-2 (HO-2) (Abbexa, Cambridge, United Kingdom) and caspase-3(Bio-Vision, Milpitas, CA, USA). All steps were accomplished as mentioned by the manufacturer. Data were calculated relative to the brain tissue protein content.
Western Blot Analysis
RIPA lysis buffer and Bradford Protein Assay Kit were used for protein determination from brain tissues (Bio Basic Inc., Markham, Ontario, Canada). The separated proteins were loaded and incubated on the PVDF membrane with primary antibodies for Beclin 1 (# PA5-20172), p-ERK (#44-680G), p-mTOR (#44-1125G), p-PI3K (# PA5-99367), ERK ( #13–6200), mTOR (# AHO1232), LC3 A (#PA5-23180), LC3 B (#PA1-16930), NF‐κB (p65) (# 51–0500), and β-actin (# MA5-15739) (Thermo Fisher Scientific Inc., Waltham. MA, USA) at 4°C. Following washing, blots were incubated with peroxidase‐labeled anti‐rabbit secondary antibodies at 37°C for 1 h. protein Bands intensity was detected using the chemiluminescent substrate (ClarityTM Western ECL substrate—Bio-Rad, TNC, USA), and a CCD camera-based imager. Analysing the images was done using Chemi Doc MP Imager and then normalised by β-actin.
Histopathological Examination and Immunohistochemistry Analysis
Histological Examination
Brain tissues were fixed in 10% neutral buffered formalin for 72 h. Afterwards, all specimens were washed with tap water, dehydrated in serial dilutions of ethanol, cleared in Xylene, and put into Paraplast tissue (Leica biosystems). Brain sections (sagittal) of 5 microns thickness were fitted on glass slides to examine cortical and hippocampal regions in various specimens. Brain tissues were then stained with Hematoxylin and Eosin (H & E) to be investigated with a light microscope. Also, other brain tissues were stained with Toluidine blue and Prussian blue to detect the mean count of intact, injured neurons and the number of ferric ion deposits, respectively. Fixation and staining of the samples were accomplished consistently with the standard procedures and protocols (Culling 2013).
Immunohistochemistry Staining and Analysis
Brain specimens were implanted in paraffin, and sections were cut to a thickness of 5 microns. Immunohistochemical staining was carried out following instructions mentioned by the manufacturer. Deparaffinisation and retrieving tissue sections were done, followed by soaking in 0.3% H2O2 for 20 min. Afterwards, brain tissues were treated with Anti– Glial Fibrillary Acidic Protein antibody as a marker for activated astrocytes (Cat. No. 13–0300- thermo scientific Co. 1:100), anti CD86 primary antibody as a marker for M1 microglial phenotype (bs-1035R, BIOSS USA – 1:150), anti CD163 antibody as a marker for M2 microglial phenotype (GeneTex. GTX35247 1:100) and Anti-Iba1 antibody as a marker for activated microglial cells (ab108539-Abcam-1:100) overnight at 4 °C. Following washing, brain tissues were incubated with a secondary antibody HRP Envision kit (DAKO) for 20 min; after that, they were incubated with diaminobenzidine (DAB) for a time interval of 15 min. Following washing, hematoxylin was used to counterstain tissues. Later, tissues were dehydrated and cleared in xylene for microscopic scanning of the cortical and hippocampal (CA1) region.
Histological Analysis
According to (Abbas et al. 2021), 6 non-overlapping fields in CA1 and cerebral cortex were chosen randomly in each sample and examined for detecting relative area percentage of immunohistochemical expression of GFAP; mean reactive microglial cells counts for CD163, CD86 and Iba-1 in immunohistochemically stained sections. Besides, the mean count of intact neurons in CA1 and cortex in toluidine blue stained tissue sections was performed. Also, the number of Ferric ion deposits was detected by Prussian blue stain in CA1 and cerebral cortex.Lastly, the optical density (OD) of CD163 expressed in neuron cells of CA1 and cortex was measured.
The Leica Application module linked to the imaging system (Leica Microsystems GmbH, Germany) was used to examine and obtain data.
Statical Analysis
Results are expressed as (mean ± standard error) by using GraphPad 9 software. Significance between groups was detected using one-way analysis of variance (ANOVA) and then Tukey’s multiple comparison test. Groups were regarded as significantly different from each other, if p value was at least < 0.05.
Results
Sert Decreased Oxidative Stress of Cerebral I/R in Rats
As illustrated in Fig. 1(a–c), cerebral I/R rats showed a significant increase of oxidative stress markers as detected by the remarkable increase of MDA, total nitrate/nitrite contents by 77%, 1.5-fold and notable decrease of GSH level by 40.38% in comparison with sham group respectively at p < 0.05. On the contrary, pre-treatment with Sert resulted in a significant decrease of MDA, and total nitrate/nitrite contents by 13, and 22.6%, respectively at p < 0.05 and an elevation of GSH level by 28% as relevant to the ischemic group at p < 0.05.
a-c Effect of Sert on oxidative stress of cerebral I/R in rats: a MDA, b Total nitrate/nitrite, c GSH content. Results were illustrated as mean ± SE (n = 6). *: significant from Sham group (p < 0.05), # significant from Sert + Sham group (p < 0.05), $: significant from I/R group (p < 0.05). Test of significance was accomplished using one way ANOVA, afterwards, Tukey’s multiple comparison test was achieved. MDA: Malonaldehyde, GSH: Glutathione
Effect of Sert on Astrocytes and Microglial Cells of Cerebral in I/R Rats
As illustrated in Fig. 2(a–f), cerebral I/R activated the microglial cells and astrocytes as confirmed by the notable increment of GFAP and Iba-1 expression in both cortex and hippocampus by 2, 1.5,3.6, 4-fold, respectively, as compared to a sham group at p < 0.05. Pre-treatment with Sert caused a remarkable decrease of GFAP expression in cortical and CA1 regions by 49.1%, 27%, respectively at p < 0.05 and a notable decline of Iba-1 expression in cortex and CA1 by 34.7%, 31.2% at p < 0.01, respectively in relevance with the ischemic group.
a-f Effect of Sert on activation of the microglial cells and astrocytes of cerebral I/R in rats: a-d GFAP, Iba-1 quantitative expression in cortex and CA1, e–f Representative photomicrography showing an increase of GFAP, and Iba-1 expression in both cortex and CA1 in I/R group as compared to sham group. The illustrated photos were magnified by × 400, with scale bars = 50 μm. Results were illustrated as mean ± SE (n = 6). *: significant from Sham group (p < 0.05), # significant from Sert + Sham group (p < 0.05), $: significant from I/R group (p < 0.05). Test of significance was accomplished using one way ANOVA, afterwards Tukey’s multiple comparison test was achieved. GFAP: Glial fibrillary acidic protein, Iba-1: Ionised calcium binding adaptor molecule 1
Sertraline Decreased Inflammation of Cerebral I/R in Rats:
As elucidated in Fig. 3(a–c), cerebral I/R in rats led to remarkable elevation of inflammatory mediators, TNF-α, IL-1 contents by 1.3-fold, 93.4%, respectively, and a significant decrease of IL-10 content by 36.2% as relevant to a sham group at p < 0.05. Interestingly, pre-treatment with Sert reversed all the previously mentioned findings as shown by the significant decrease of TNF-α, and IL-1 levels by 19, and 12% respectively at p < 0.05 and enhancement of IL-10 content by 17% at p < 0.05 as relevant to ischemic group.
a-c Effect of Sert on inflammation predisposed by cerebral I/R in whole brain tissues of rats: a TNF-α, b IL-1, and c IL-10 content. Results were illustrated as mean ± SD (n = 6). *: significant from Sham group (p < 0.05), # significant from Sert + Sham group (p < 0.05), $: significant from I/R group (p < 0.05). Test of significance was accomplished using one way ANOVA, afterwards Tukey’s multiple comparison test was achieved. IL: Interleukin, TNF-α: Tumor necrosis factor-alpha
Effect of Sert on Phenotype of Microglial Cell of Cerebral I/R in Rats via Involvement of NF-ĸβ, and p-ERK
As illustrated in Fig. 4(a–i), cerebral I/R in rats resulted in a notable increase of NF-ĸB P65 and p-ERK expression by 4.6, and 4.7-fold, respectively at p < 0.05. This led to M1 phenotype activation as indicated by increased expression of CD 86 in both cortex and CA1 region by 9.2, and 5.3-fold, respectively in comparison with the sham group, at p < 0.05. Conversely, pre-treatment with Sert significantly decreased expression of NF-ĸB P65 and p-ERK by 55.8,62.8%, respectively at p < 0.05 as compared to I/R group, hence shifting microglial cells to M2 phase as evidenced by a remarkable decrease of CD 86 by 58.7, 77.2%, respectively and increase of CD 163 expression in both cortical and CA1 regions by 54%, 2.8-fold, respectively in comparison with the ischemic group respectively at p < 0.05.
a-i: Effect of Sert on microglial/macrophage polarisation of cerebral I/R in rats: a-b Quantitative expression of NF-ĸBp65, p-ERK/t-ERK, c Representative western blot bands of NF-ĸb p65 and p-ERK expression in different treatment groups, d-g Quantitative CD 163 and CD 86 expression in both cortical and CA1 regions for different treatment groups, h-i Representative photomicrography displaying CD86 and CD 163 expression in both regions, cortex and CA1 for different treatment groups. The illustrated photos were magnified by × 400, with scale bars = 50 μm. Results were illustrated as mean ± SE (n = 6). *: significant from Sham group (p < 0.05), # significant from Sert + Sham group (p < 0.05), $: significant from I/R group (p < 0.05). Test of significance was accomplished using one-way ANOVA, afterwards Tukey’s multiple comparison test was achieved. CD: Cluster of differentiation, ERK1/2: Extracellular signal related kinases, NF-ĸBp65: Nuclear factor kappa B
Effect of Sert on Ischemic Stroke Transformation to Haemorrhagic Stroke of Cerebral I/R in Rats
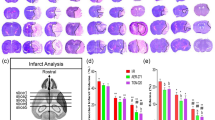
As elucidated in Fig. 5(a–i), cerebral I/R in rats induced transformation of ischemic stroke into haemorrhagic stroke as evidenced by a remarkable increment of CD 163 expression in neuron cells of both cortex and CA1 region by 10.6- fold, 84%, respectively in comparison with the sham group at p < 0.05. Contrarily, Sert remarkably halted haemorrhagic transformation (HT) by significantly decreasing the expression of CD 163 in neuron cells of both regions, cortex and CA1 by 64, 21% as compared to the ischemic group at p < 0.05, respectively. This was evidenced by measuring the levels of MMP-2,9, which are responsible for blood membrane barrier integrity. Cerebral I/R displayed an increment increase in the level of MMP-2,9 by 1.4, and 1.6-fold, respectively as compared to the sham group at p < 0.05. On the other hand, pre-treatment with Sert reversed that by a significant decrease in levels of MMP-2,9 by 45.3,47.3%, respectively as compared to the I/R group.
a-i Effect of Sert on the transformation of ischemic stroke to haemorrhagic of cerebral I/R in rats: a-b Quantitative neuronal CD 163 protein expression in both cortex and CA1, c Representative photomicrography displaying the expression of neuronal CD 163 in cortical and CA1 regions for different treatment groups, d-e level of MMP-9, MMP-2 for the different treated group, f activity of heme oxygenase-2 for all treated groups, g representative photomicrography for ferric ion deposits in both cortex and CA1 region, and h-i the number of Ferric ion deposits in both cortex and CA1region. The illustrated photos were magnified by × 400, with scale bars = 50 μm. Results were illustrated as mean ± SE (n = 6). *: significant from Sham group (p < 0.05), # significant from Sert + Sham group (p < 0.05), $: significant from I/R group (p < 0.05). Test of significance was accomplished using one way ANOVA, afterwards Tukey–Kramer multiple comparison test was achieved. CD: Cluster of differentiation
Moreover, it was found that the activity of HO-2 significantly increased in I/R by 2.4-fold compared to the sham group at p < 0.05. This is a clue for the presence of haemoglobin in the extracellular matrix between neurons due to haemorrhagic transformation. However, pre-treatment converted this situation by a significant decrease in the activity of HO-2 by 36.6% compared to the I/R group.
Finally, the previously mentioned results were further confirmed by the Prussian blue stain, which counts the number of ferric ion deposits in the field due to the lysis of red blood corpuscles (RBCs) and release of the heme group. In the I/R group, there was a significant increase in the number of ferric ion deposits in both cortex and CA1 by 4.8, and 12.6 -fold, respectively at p < 0.05 as compared to the sham group. On the other hand, pre-treatment with Sert decreased the number of ferric ion deposits in both cortex and CA1 by 37, and 36.6%, respectively at p < 0.05 as compared to the I/R group.
Sert Decreased Autophagy of Cerebral I/R in Rats
As illustrated in Fig. 6(a–e), autophagy was enhanced in rats exposed to cerebral I/R as detected by the significant decline in p-PI3K, p-mTOR expression by 69, 82%, respectively and increase in expression of Beclin-1, LC-3II/I by 4.7-fold, 54%, respectively as compared by sham group at p < 0.05. Contrarily, pre-treatment with Sert remarkably suppressed autophagy as manifested by the significant decrease of Beclin-1, LC3-II/I expression by 54.3, 34%, respectively and increased expression of p-PI3K, p-mTOR by 1.45, 2.7-fold, respectively as relevant to I/R group at p < 0.05.
a-e Effect of Sert on autophagy of cerebral I/R in rats: a-d) Quantitative p-PI3K, p-mTOR, Beclin-1 and LC3II/I protein expression, e) Representative western blot bands showing expression of p-PI3K, p-mTOR, Beclin-1 and LC3II/I in different treatment groups. Results were illustrated as mean ± SE (n = 6). *: significant from Sham group (p < 0.05), # significant from Sert + Sham group (p < 0.05), $: significant from I/R group (p < 0.05). Test of significance was accomplished using one way ANOVA, afterwards Tukey–Kramer multiple comparison test was achieved. p-mTOR: phosphorylated Mammalian target of rapamycin, p-PI3K: phosphorylated Phosphoinositide 3-kinases
Effect of Sert on Bax, Bcl-2, Caspase-3 Contents and Neuronal Density of Cerebral in I/R Rats
Figure 7(a–f) illustrates the apoptotic induction in cerebral I/R in rats as evidenced by the significant increment of Bax, and Caspase-3 contents by 1-fold, 78%, respectively, reduction of Bcl-2 level by 28.3% as well as decreased intact neuron cell number in both cortex and CA1 by 96.7,43% respectively as compared to the sham group at p < 0.05. However, pre-treatment with Sert induced neuronal survival as evidenced by a remarkable decrease of Bax, Caspase-3 levels by 20.4%,19.5%, increased content of Bcl-2 by 14.3%, and enhanced intact neuron cell number in both cortical and CA1 regions by 26-fold, 40%, respectively at p < 0.05.
a-f Effect of Sert on Bax, Bcl-2, caspase-3 contents and neuronal density of cerebral I/R in rats: a-e Quantitative expression of Bax, Bcl-2, caspase -3, number of intact neurons in both region cortex and CA1, f Representative photomicrography displaying the number of intact neurons in both cortex, CA1 in different treatment groups after staining with toluidine blue. The illustrated photos were magnified by × 400, with scale bars = 50 μm. Results were illustrated as mean ± SE (n = 6). *: significant from Sham group (p < 0.05), # significant from Sert + Sham group (p < 0.05), $: significant from I/R group (p < 0.05).Test of significance was accomplished using one way ANOVA, afterwards Tukey’s multiple comparison test was achieved. Bcl-2: B-cell lymphoma 2, Bax: BCL2 Associated X
Effect of Sert on Histological Changes of Cerebral I/R in Rats
Sham and Sert cortical samples displayed a standard histological feature of cerebral cortical layers. The I/R group showed severe degenerative neurons and high reactive glial cell infiltration.On the other hand, pre-treatment with Sert showed attenuation in neuronal damage, and many well-organised neurons with milder glial cells infiltrate. Also, Sham and Sert CA1 samples showed typically organised histological features of hippocampal layers arrow.The I/R group showed many degenerated neurons, fewer degrees of intact pyramidal neurons and a moderately higher degree of reactive glial cell infiltrates.The pretreated group displayed a few neuronal degenerative changes and many well-organised neurons accompanied by mild infiltration of glial cells (arrowhead) (Fig. 8).
Representative photomicrography showing Sham and Sert cortical samples displaying typical histological features (black arrow). The I/R group showed severe degeneration of neurons (red arrow) and a high degree of reactive glial cell infiltration (arrowhead).On the contrary, pre-treatment with Sert showed attenuation in neuronal damage (red arrow), and many well-organised neurons (black arrow) with milder glial cell infiltrates (arrowhead). Also, Sham and Sert CA1 samples showed standard organised histological features of hippocampal layers (black arrow). I/R group showed many degenerated neurons (red arrow), fewer degrees of intact pyramidal neurons (black arrow) and moderate higher degree of reactive glial cells infiltrates (arrowhead). The pretreated group displayed a few neuronal degenerative changes (red arrow) and many well-organised neurons (black arrow) accompanied with mild infiltration of glial cells (arrowhead). The illustrated photos were magnified by × 400, with scale bars = 50 μm
Discussion
Cerebral I/R injury is a complicated neurodegenerative disease that leads to serious outcomes and affects individuals’ lives (Wu et al. 2022). The aim of the current was to elucidate the protective effect of Sert against cerebral I/R injury in rats. Oxidative stress is a mainstay pathophysiological mechanism of cerebral injury, which occurs due to the over-liberation of reactive oxygen species (ROS), resulting in an imbalance of oxidant-antioxidant equilibrium in favour of oxidation, and consequently, mitochondrial dysfunction occurs. Mitochondrial dysfunction itself acts as a ROS generator, resulting in a vicious cycle of ROS generation (Wu et al. 2020; Mei et al. 2022).
Consistent with previous studies (Zhang and Cui 2022; Yang et al. 2022), our results showed increased oxidative stress in the cerebral I/R group as manifested by elevated MDA, total nitrate/ nitrite contents and decreased GSH level.
Moreover, inflammation is another deleterious pathophysiological event involved in cerebral deterioration. It is documented that up-regulation of neuroinflammatory mediators in cerebral I/R causes persistent damage to the brain, which plays a crucial role in the injury of neurons occurring in the acute phase of ischemic stroke (Zhao et al. 2020; Nie et al. 2022). This pathway leads to the activation of glial cells through multiple molecular pathways as activated NF-ĸB p65. Glial cells preserve the integrity of the brain tissue and protect it from injury resulting from a dangerous insult. It was stated that microglial cells, representing about 15% of brain cells, are in a resident state until activated by noxious factors as pathogen or neurodegenerative disease. Microglial cells act as a first defence line for CNS microenvironment, which is activated minutes to a few hours following ischemia. In the same context, activation of microglial cells is detected by measurement of Iba-1 expression (Hernández et al. 2021). Additionally, it was found that astrocytes account for 50% of the brain volume. The main function of astrocytes is to maintain the efficient processing of brain tissue by balancing ion-water content, downregulating excitatory neurotransmitters and getting rid of wastes. Astrocyte activation occurs during cerebral I/R, leading to astrogliosis that is detected by GFAP. (Hernández et al. 2021; Jurcau and Simion 2022).
In our study, cerebral I/R resulted in enhanced NF-ĸB p65, GFAP, and Iba-1 protein expression, along with enhanced IL-1, TNF-α levels and decreased IL-10 content. Earlier studies showed that in the cerebral I/R model, oxidative stress enhances transcription factors such as NF-ĸB, which is distributed in the neurons, microglial cells, and astrocytes. This leads to the activation of astrocytes and microglial cells with the subsequent overproduction of inflammatory mediators and a decrease in the production of anti-inflammatory mediators (Zhou et al. 2018; Jurcau and Simion 2022).
The microglial cells activation to classic (M1) or alternative (M2) phenotypes is considered as a macrophage response which occurs due to ischemia. M1 phenotype acts as an indicator of the inflammatory phase by releasing inflammatory cytokines such as IL-1, TNF-α. Many upstream regulatory cascades lead to M1 activation, such as the ERK pathway, activated NF-Kβ p65 and IL-1. Conversely, the M2 phenotype indicates the anti-inflammatory phase, leading to enhanced release of anti-inflammatory cytokines as IL-10 (Zhao et al. 2017).
In the same context, the current data showed a remarkable increase in the expression of ERK, NF-Kβ p65 and CD 86 (M1 marker of glial cells) of the cerebral I/R group. Previous results showed that microglial/microphage polarisation to the proinflammatory M1 phenotype in the cerebral I/R model was attributed to oxidative stress, upregulation of NF-KB and ERK (Gaire et al. 2019; Yang et al. 2019; Jurcau and Simion 2022).
Ischemic stroke can be converted to haemorrhagic stroke due to multiple factors such as ROS and inflammation (Shao et al. 2021; Spronk et al. 2021). During haemorrhagic stroke, lysis of red blood cells occurs. Released haemoglobin (Hb), which participates in the aggravation of oxidative stress, is bound to haptoglobin, forming a complex that binds to CD 163 (a receptor found on neuron cells) and is engulfed by macrophages. Consequently, the upregulation of CD 163 is an indisputable indication of the occurrence of haemorrhagic stroke (Garton et al. 2017). Moreover, a previous study revealed that the expression of CD 163 in neurons is increased after intracranial haemorrhage (ICH) to quarantine the hemolytic product of red blood cells (Hb) and decrease the arising inflammatory cascade as well as generation of ROS. It was found that CD163 expression is increased in both hematoma and perihematomal regions within 6 h after ICH (Garton et al. 2017; Liu et al. 2017).
In our study, Ischemic stroke was transformed into haemorrhagic stroke in the ischemic group, as evidenced by enhanced CD 163 expression in neuron cells. It is noteworthy that haemorrhagic stroke plays a crucial role in maintaining microglial cells in the proinflammatory M1 phase through NF-ĸB-p65 upregulation and ERK phosphorylation (Bi et al. 2021).
In the present study, it was found that levels of MMP-2,9 elevated in I/R group as compared to the sham group. Conversion of ischemic stroke to haemorrhagic stroke was evidenced by measuring matrix metalloproteinase enzymes (MMPs), which have a fundamental role in disrupting BBB via degrading tight junction proteins, resulting in the release of RBCs (Spronk et al. 2021). Previous studies documented that MMP-2 and MMP-9 are essential in BBB disruption during ischemic stroke. It was found that MMP-2 is the initiator of BBB derangement following cerebral ischemia, while MMP-9 is the principal contributor to BBB dysregulation in the delayed phase after ischemic stroke (Lakhan et al. 2013). Moreover, it was demonstrated that upregulated levels of MMPs in the cerebral ischemia group resulted from the generation of reactive oxygen and nitrogen species following reperfusion, leading to haemorrhagic transformation (HT) (Hong et al. 2021). In addition, the cerebral I/R group significantly enhanced HO-2 activity compared to the sham group.
As mentioned before, after leakage, RBCs undergo lysis with the subsequent release of Hb molecules, which are engulfed by the neuronal cells via binding to the neuronal receptor CD163. In the presence of HO-2, an enzyme that is mainly expressed in the neurons, Hb molecules are degraded, leading to the release of intracellular iron, which gives rise to reactive oxygen species (Garton et al. 2017; Liu et al. 2017; Ma et al. 2016). In the same context, as an undisputable confirmation of induction of HT, the Prussian blue stain revealed a significant upregulation of ferric ion deposits in brain tissue as a result of HT in I/R compared to the sham group.
Autophagy plays a severe role in injury caused to neurons due to cerebral I/R. Autophagy is mainly initiated by the liberation of ROS that leads to the downregulation of phosphorylation of PI3K with the consequent inhibition of the downstream mTOR, an essential autophagy regulatory protein. Inhibition of mTOR increases the relative expression of autophagic mediators as LC3 II/I and Beclin-1 (Yan et al. 2019; Yang et al 2019; Zhao et al. 2022). LC3 is present on the autophagosome membrane and is engaged in forming an intact autophagosome. In addition, Beclin-1 is critically involved in lysosome fusion and autophagy initiation (Wei et al. 2021). Moreover, many studies noted that autophagic upregulation is accompanied by apoptotic enhancement (Li et al. 2015; Chen et al. 2022). In our study, PI3K, mTOR, Beclin-1 and LC3 II/I expression were elevated in the cerebral I/R group, along with increased levels of apoptotic makers, caspase-3, Bax, decrease of Bcl-2 content, as well as downregulation of intact neurons number in both cortex and hippocampal CA1 region.
Pre-treatment with Sert exhibited a protective effect against the cerebral I/R model, as shown by decreased MDA, total nitrate/nitrite levels and enhanced GSH content as relevant to the cerebral I/R group. Conforming with our results, a previous study showed that Sert decreased oxidative stress markers in heart failure patients (Michalakeas et al. 2011).
Moreover, Sert down-regulated NF-ĸB-p65, p-ERK, GFAP, Iba-1 expression as well as IL-1, TNF-α contents and increased IL-10 content as relevant to the cerebral I/R group. These outcomes were confirmed by previous studies, which reported that Sert prevented the activation of microglial cells by suppressing NF-ĸB expression (Sitges et al. 2014; Lu et al. 2019).
In addition, pre-treatment with Sert shifted activated microglial cells from the M1 phenotype to the anti-inflammatory M2 phenotype, as detected by increased CD163 and decreased CD 86 expression in comparison with the cerebral I/R group. It was previously documented that M2 polarisation is attributed to many factors, such as decreased activation of ERK, mitigated liberation of ROS and suppressed NF-ĸB-p65 expression (Su et al. 2015; Yang et al. 2019). In the same context, our study showed that Sert inhibits haemorrhagic transformation as detected by suppressed expression of CD163 in neurons, MMP-2,9 levels, ferric ion deposits and activity of HO-2 as compared to the cerebral I/R group.
Finally, compared to the cerebral I/R group, Sert enhanced PI3K and mTOR expression, leading to inhibition of autophagy as indicated by decreased Beclin-1 and LC3II/I expression. Moreover, in consistency with a previous study (Wann et al. 2009), apoptosis was inhibited as demonstrated by the decline of apoptotic markers, Bax, caspase-3, enhancement of anti-apoptotic marker, Bcl-2, and restoration of the number of intact neuron cells. It is worth mentioning that all the aforementioned results were further confirmed by histological examination.
Conclusion
Our study revealed that Sert has a protective effect against cerebral I/R via multiple pathways, including inhibition of oxidative stress, increased polarisation of microglial cells to the anti-inflammatory M2 phenotype and hence, suppression of inflammation. Moreover, Sert reduced autophagy and apoptosis. Interestingly, our study showed that Sert inhibited haemorrhagic transformation through downregulation of neuronal CD163 expression, attenuation of MMP-2, MMP-9 levels and HO-2 activity.
Data Availability
All data obtained from this study are presented in the paper. Raw data are available on request.
References
Abbas H, Gad HA, Khattab MA, Mansour M (2021) The tragedy of Alzheimer’s disease: Towards better management via resveratrol-loaded oral bilosomes. Pharmaceutics 13:1635. https://doi.org/10.3390/pharmaceutics13101635
Atli O, Baysal M, Aydogan-Kilic G, Kilic V, Ucarcan S, Karaduman B, Ilgin S (2017) Sertraline-induced reproductive toxicity in male rats: Evaluation of possible underlying mechanisms. Asian J Androl 19(6):672–679. https://doi.org/10.4103/1008-682X.192637
Baharav E, Bar M, Taler M, Gil-Ad I, Karp L, Weinberger A, Weizman A (2012) Immunomodulatory effect of sertraline in a rat model of rheumatoid arthritis. NeuroImmunoModulation 19:309–318. https://doi.org/10.1159/000339109
Bi R, Fang Z, You M, He Q, Hu B (2021) Microglia phenotype and intracerebral hemorrhage: A balance of yin and yang. Front Cell Neurosci 15. https://doi.org/10.3389/fncel.2021.765205
Chen S, Zhang S, Wu H, Zhang D, You G, You J, Zheng N (2022) Protective effect of phillyrin against cerebral ischemia/reperfusion injury in rats and oxidative stress-induced cell apoptosis and autophagy in neurons. Bioengineered 13(3):7940–7950. https://doi.org/10.1080/21655979.2022.2042142
Culling CFA (2013) Handbook of Histopathological and Histochemical Techniques. Ed. 3, Butterworths, London, UK
Dong R, Huang R, Wang J, Liu H, Xu Z (2021) Effects of microglial activation and polarization on brain injury after stroke. Front Neurol 1(12):620948. https://doi.org/10.3389/fneur.2021.620948. PMID: 34276530; PMCID: PMC8280287
Fernandez-Lizarbe S, Pascual M, Guerri C (2009) Critical role of TLR4 response in the activation of microglia by ethanol. J Immunol 183:4733–4744
Gaire BP, Bae YJ, Choi JW (2019) S1P1 regulates M1/M2 polarization toward brain injury after transient focal cerebral ischemia. Biomol Ther (Seoul) 27(6):522–529. https://doi.org/10.4062/biomolther.2019.005
Garton T, Keep RF, Hua Y, Xi G (2017) CD163, a hemoglobin/haptoglobin scavenger receptor, after intracerebral hemorrhage: Functions in microglia/macrophages versus neurons. Transl Stroke Res 8:612–616. https://doi.org/10.1007/s12975-017-0535-5
Hernández IH, Villa-González M, Martín G, Soto M, Pérez-Álvarez MJ (2021) Glial cells as therapeutic approaches in brain ischemia-reperfusion injury. Cells 10(7):1639. https://doi.org/10.3390/cells10071639
Hong JM, Kim DS, Kim M (2021) Hemorrhagic transformation after ischemic stroke: Mechanisms and management. Front Neurol 12:703258. https://doi.org/10.3389/fneur.2021.703258
Jiang CT, Wu WF, Deng YH, Ge JW (2020) Modulators of microglia activation and polarisation in ischemic stroke (Review). Mol Med Rep 21(5):2006–2018. https://doi.org/10.3892/mmr.2020.11003. Epub 2020 Feb 26. PMID: 32323760; PMCID: PMC7115206
Jin Z, Guo P, Li X, Ke J, Wang Y, Wu H (2019) Neuroprotective effects of irisin against cerebral ischemia/ reperfusion injury via Notch signaling pathway. Biomed Pharmacother 120:109452. https://doi.org/10.1016/j.biopha.2019.109452
Jurcau A, Simion A (2022) Neuroinflammation in cerebral ischemia and ischemia/reperfusion injuries: From pathophysiology to therapeutic strategies. Int J Mol Sci 23:14. https://doi.org/10.3390/ijms23010014
Lakhan SE, Kirchgessner A, Tepper D, Leonard A (2013) Matrix metalloproteinases and blood-brain barrier disruption in acute ischemic stroke. Front Neurol 4:32. https://doi.org/10.3389/fneur.2013.00032
Leclerc JL, Lampert AS, Loyola Amador C, Schlakman B, Vasilopoulos T, Svendsen P, Moestrup SK, Doré S (2018) The absence of the CD163 receptor has distinct temporal influences on intracerebral hemorrhage outcomes. J Cereb Blood Flow Metab 38(2):262–273. https://doi.org/10.1177/0271678X17701459. Epub 2017 Mar 30. PMID: 28358264; PMCID: PMC5951015
Li H, Liu X, Zhu Y, Liu Y, Wang Y (2015) Magnolol derivative 002C–3 protects brain against ischemia-reperfusion injury via inhibiting apoptosis and autophagy. Neurosci Lett 588:178–183. https://doi.org/10.1016/j.neulet.2015.01.007
Liu R, Cao S, Hua Y, Keep RF, Huang Y, Xi G (2017) CD163 expression in neurons after experimental intracerebral hemorrhage. Stroke 48(5):1369–1375. https://doi.org/10.1161/STROKEAHA.117.016850
Lu Y, Xu X, Jiang T, Jin L, Zhao XD, Cheng JH, Jin XJ, Ma J, Piao HN, Piao LX (2019) Sertraline ameliorates inflammation in CUMS mice and inhibits TNF-α-induced inflammation in microglia cells. Inter Immunopharmacol 67:119–128. https://doi.org/10.1016/j.intimp.2018.12.011
Ma B, Day JP, Phillips H, Slootsky B, Tolosano E, Dorѐ S (2016) Deletion of the hemopexin or heme oxygenase-2 gene aggravates brain injury following stroma-free hemoglobin-induced intracerebral hemorrhage. J Neuroinflammation 13:26. https://doi.org/10.1186/s12974-016-0490-1
Mei Z, Du L, Liu X, Chen X, Tian H, Deng Y, Zhang W (2022) Diosmetin alleviated cerebral ischemia/reperfusion injury in vivo and in vitro by inhibiting oxidative stress via the SIRT1/Nrf2 signaling pathway. Food Funct 4;13(1):198–212. https://doi.org/10.1039/d1fo02579a
Michalakeas CA, Parissis JT, Douzenis A, Nikolaou M, Varounis C, Andreadou I, Antonellos N, Markantonis-Kiroudis S, Paraskevaidis I, Ikonomidis I, Lykouras E, Kremastinos D (2011) Effects of sertraline on circulating markers of oxidative stress in depressed patients with chronic heart failure: A pilot study. J Card Fail 17(9):748–754. https://doi.org/10.1016/j.cardfail.2011.05.004
Nie QQ, Zheng ZQ, Liao J, Li YC, Chen YT, Wang TY, Yuan GQ, Wang Z, Xue Q (2022) SPP1/AnxA1/TIMP1 as essential genes regulate the inflammatory response in the acute phase of cerebral ischemia-reperfusion in rats. J Inflamm Res 15:4873–4890. https://doi.org/10.2147/JIR.S369690
Ozturk M, Ucar S, SarJ F, Erdogan S, Topdag M, Iseri M (2013) Possible protective effect of sertraline against cisplatin-induced ototoxicity: An experimental study. Sci World J Vol 2013. https://doi.org/10.1155/2013/523480
Shao A, Gao A, Wu H, Xu W, Pan Y, Fang Y, Wang X, Zhang J (2021) Melatonin ameliorates hemorrhagic transformation via suppression of ROS-induced NLRP3 activation after cerebral ischemia in hyperglycemic rats. Oxid Med Cell Longev. https://doi.org/10.1155/2021/6659282
Shin TK, Kang MS, Lee HY, Seo MS, Kim SG, Kim CD, Lee WS (2009) Fluoxetine and sertraline attenuate postischemic brain injury in mice. Korean J Physiol Pharmacol 13(3):257–63. https://doi.org/10.4196/kjpp.2009.13.3.257
Sitges M, Gómez CD, Aldana BI (2014) Sertraline reduces IL-1β and TNF-α mRNA expression and overcomes their rise induced by seizures in the rat hippocampus. PLoS One 9(11):e111665. https://doi.org/10.1371/journal.pone.0111665
Spronk E, Sykes G, Falcione S, Munsterman D, Joy T, Kamtchum-Tatuene J, Jickling GC (2021) Hemorrhagic transformation in ischemic stroke and the role of inflammation. Front Neurol 12:661955. https://doi.org/10.3389/fneur.2021.661955
Su F, Yi H, Xu L, Zhang Z (2015) Fluoxetine and S-citalopram inhibit M1 activation and promote M2 activation of microglia in vitro. Neuroscience 294:60–8. https://doi.org/10.1016/j.neuroscience.2015.02.028
Sun X, Wang D, Zhang T, Lu X, Duan F, Ju L, Zhuang X, Jiang X (2020) Eugenol attenuates cerebral ischemia-reperfusion injury by enhancing autophagy via AMPK-mTOR-P70S6K pathway. Front Pharmacol 11. https://doi.org/10.3389/fphar.2020.00084
Wanchao S, Chen M, Zhiguo S, Futang X, Mengmeng S (2018) Protective effect and mechanism of Lactobacillus on cerebral ischemia reperfusion injury in rats. Braz J Med Biol Res 51(7):e7172. https://doi.org/10.1590/1414-431x20187172
Wang J, Mao J, Wang R, Li S, Wu B, Yuan Y (2020) Kaempferol protects against cerebral ischemia reperfusion injury through intervening oxidative and inflammatory stress induced apoptosis. Front Pharmacol 11:424. https://doi.org/10.3389/fphar.2020.00424
Wann BP, Bah TM, Kaloustian S, Boucher M, Dufort AM, Le Marec N, Godbout R, Rousseau G (2009) Behavioural signs of depression and apoptosis in the limbic system following myocardial infarction: Effects of sertraline. J Psychopharmacol 23(4):451–9. https://doi.org/10.1177/0269881108089820
Wei W, Min L, Xiao-Bing L, Ning L, Wei-Ke S, Alexandr VD, Jian-Qiang Y (2021) Neuroprotective effects of oxymatrine on PI3K/Akt/mTOR pathway after hypoxic-ischemic brain damage in neonatal rats. Front Pharmacol 12. https://doi.org/10.3389/fphar.2021.642415
Wu F, Liu Z, Zhou L, Ye D, Zhu Y, Huang K, Weng Y, Xiong X, Zhan R, Shen J (2022) Systemic immune responses after ischemic stroke: From the center to the periphery. Front Immunol 13:911661. https://doi.org/10.3389/fimmu.2022.911661. PMID: 36211352; PMCID: PMC9533176
Wu L, Xiong X, Wu X, Ye Y, Jian Z, Zhi Z, Gu L (2020) Targeting oxidative stress and inflammation to prevent ischemia-reperfusion injury. Front Mol Neurosci 5(13):28. https://doi.org/10.3389/fnmol.2020.00028
Wu R, Li X, Xu P, Huang L, Cheng J, Huang X, Jiang J, Wu LJ, Tang Y (2017) TREM2 protects against cerebral ischemia/reperfusion injury. Mol Brain 10:20. https://doi.org/10.1186/s13041-017-0296-9
Xu S, Lu J, Shao A, Zhang JH, Zhang J (2020) Glial cells: Role of the immune response in ischemic stroke. Front in Immunol 11:294. https://doi.org/10.3389/fimmu.2020.00294
Yan BC, Wang J, Rui Y, Cao J, Xu P, Jiang D, Zhu X, Won MH, Bo P, Su P (2019) Neuroprotective effects of gabapentin against cerebral ischemia reperfusion-induced neuronal autophagic injury via regulation of the PI3K/Akt/mTOR signaling pathways. J Neuropathol Exp Neurol 78(2):157–171. https://doi.org/10.1093/jnen/nly119
Yang S, Wang H, Yang Y, Wang R, Wang Y, Wu C, Du G (2019) Baicalein administered in the subacute phase ameliorates ischemia-reperfusion-induced brain injury by reducing neuroinflammation and neuronal damage. Biomed Pharmacother 117:109102. https://doi.org/10.1016/j.biopha.2019.109102
Yang Y, He B, Zhang X, Yang R, Xia X, Chen L, Li R, Shen Z, Chen P (2022) Geraniin protects against cerebral ischemia/reperfusion injury by suppressing oxidative stress and neuronal apoptosis via regulation of the Nrf2/HO-1 pathway. Oxid Med Cell Longev 2022:2152746. https://doi.org/10.1155/2022/2152746
Yu L, Su X, Li S, Zhao F, Mu D, Qu Y (2020) Microglia and their promising role in ischemic brain injuries: An update. Front Cell Neurosci 14:211. https://doi.org/10.3389/fncel.2020.00211
Zhang H, Lu M, Zhang X, Kuai Y, Mei Y, Tan Q, Zhong K, Sun X, Ta W (2019) Isosteviol sodium protects against ischemic stroke by modulating microglia/macrophage polarization via disruption of GAS5/miR-146a-5psponge. Sci Rep 9:12221. https://doi.org/10.1038/s41598-019-48759-0
Zhang Y, Cao Y, Liu C (2020) Autophagy and ischemic stroke. Adv Exp Med Biol 1207:111–134. https://doi.org/10.1007/978-981-15-4272-5_7
Zhang P, Cui J (2022) Neuroprotective effect of fisetin against the cerebral ischemia-reperfusion damage via suppression of oxidative stress and inflammatory parameters. Inflammation 44:1490–1506. https://doi.org/10.1007/s10753-021-01434-x
Zhao SC, Ma LS, Chu ZH, Xu H, Wu WQ, Liu F (2017) Regulation of microglial activation in stroke. Acta Pharmacol Sin 38(4):445–458. https://doi.org/10.1038/aps.2016.162
Zhao J, Piao X, Wu Y, Liang S, Han F, Liang Q, Shao S, Zhao D (2020) Cepharanthine attenuates cerebral ischemia/reperfusion injury by reducing NLRP3 inflammasome-induced inflammation and oxidative stress via inhibiting 12/15-LOX signaling. Biomed Pharmacother 127:110151. https://doi.org/10.1016/j.biopha.2020.110151
Zhao T, Lu H, Li M, Yan Q, Gu J, Liu L (2022) Neuroprotective mechanism of crocin via PI3K/Akt/mTOR signaling pathway after cerebral infarction: an in vitro study. Am J Transl Res 15;14(5):3164–3171
Zhou J, Li M, Jin W-F, Li X-H, Zhang Y-Y (2018) Role of NF-κB on neurons after cerebral ischemia reperfusion. Int J Pharmacol 14:451–459. https://doi.org/10.3923/ijp.2018.451.459
Acknowledgements
The authors would like to express their deep appreciation to Dr. Mohamed Abderazik Khattab, Department of Cytology and Histology, Faculty of Veterinary Medicine, Cairo University for his unfailing efforts regarding the clarification of the histological slides.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). Authors declared that they did not receive any financial support from any agency.
Author information
Authors and Affiliations
Contributions
Shimaa Kamal Mohamed: Conceptualization, Methodology, Investigation, Data curation, Formal analysis, Writing – original draft, Funding acquisition. Abeer Elkhoely: Supervision, investigation, Writing – review & editing, Visualization, Project administration, Funding acquisition. Amany Ali Eissa Ahmed: Supervision, Funding acquisition. The descriptions are accurate and agreed by all authors.
Corresponding author
Ethics declarations
Ethical Approval
The protocol is approved by the ethical committee of Helwan university. Animals caring and handling of animals were adhered to the committee guidelines, which follows the guidelines of ARRIVES.
Consent to Participate
Not available.
Consent to Publication
Not available.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mohamed, S.K., Ahmed, A.A.E. & Elkhoely, A. Sertraline Pre-Treatment Attenuates Hemorrhagic Transformation Induced in Rats after Cerebral Ischemia Reperfusion via Down Regulation of Neuronal CD163: Involvement of M1/M2 Polarization Interchange and Inhibiting Autophagy. J Neuroimmune Pharmacol 18, 657–673 (2023). https://doi.org/10.1007/s11481-023-10093-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-023-10093-8