Abstract
Adult-onset neurodegenerative disorders, like Parkinson’s disease (PD) and dementia with Lewy bodies (DLB), that share the accumulation of aggregated α-synuclein (αSynagg) as their hallmark molecular pathology are collectively known as α-synucleinopathies. Diagnosing α-synucleinopathies requires the post-mortem detection of αSynagg in various brain regions. Recent efforts to measure αSynagg in living patients include quantifying αSynagg in different biofluids as a biomarker for PD. We adopted the real-time quaking-induced conversion (RT-QuIC) assay to detect very low levels of αSynagg. We first optimized RT-QuIC for sensitivity, specificity, and reproducibility by using monomeric recombinant human wild-type αSyn as a substrate and αSynagg as the seed. Next, we exposed mouse microglia to αSyn pre-formed fibrils (αSynPFF) for 24 h. RT-QuIC assay revealed that the αSynPFF is taken up rapidly by mouse microglia, within 30 min, and cleared within 24 h. We then evaluated the αSyn RT-QuIC assay for detecting αSynagg in human PD, DLB, and Alzheimer's disease (AD) post-mortem brain homogenates (BH) along with PD and progressive supranuclear palsy (PSP) cerebrospinal fluid (CSF) samples and then determined protein aggregation rate (PAR) for αSynagg. The PD and DLB BH samples not only showed significantly higher αSynagg PAR compared to age-matched healthy controls and AD, but RT-QuIC was also highly reproducible with 94% sensitivity and 100% specificity. Similarly, PD CSF samples demonstrated significantly higher αSynagg PAR compared to age-matched healthy controls, with 100% sensitivity and specificity. Overall, the RT-QuIC assay accurately detects αSynagg seeding activity, offering a potential tool for antemortem diagnosis of α-synucleinopathies and other protein-misfolding disorders.

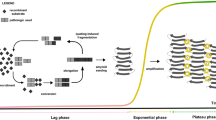
A schematic representation of αSyn RT-QuIC assay.







Similar content being viewed by others
References
Allen Reish HE, Standaert DG (2015) Role of α-synuclein in inducing innate and adaptive immunity in Parkinson disease. J Parkinsons Dis 5:1–19
Beach TG, Adler CH (2018) Importance of low diagnostic accuracy for early Parkinson's disease. Mov Disord 33:1551–1554
Bidinosti M, Shimshek DR, Mollenhauer B, Marcellin D, Schweizer T, Lotz GP, Schlossmacher MG, Weiss A (2012) Novel one-step immunoassays to quantify alpha-synuclein: applications for biomarker development and high-throughput screening. J Biol Chem 287:33691–33705
Block ML, Zecca L, Hong JS (2007) Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 8:57–69
Braak H, Braak E (2000) Pathoanatomy of Parkinson's disease. J Neurol 247(Suppl 2):Ii3–I10
Braak H, Bohl JR, Muller CM, Rub U, de Vos RA, Del Tredici K (2006) Stanley Fahn lecture 2005: the staging procedure for the inclusion body pathology associated with sporadic Parkinson's disease reconsidered. Mov Disord 21:2042–2051
Compta Y, Valente T, Saura J, Segura B, Iranzo A, Serradell M, Junque C, Tolosa E, Valldeoriola F, Munoz E, Santamaria J, Camara A, Fernandez M, Fortea J, Buongiorno M, Molinuevo JL, Bargallo N, Marti MJ (2015) Correlates of cerebrospinal fluid levels of oligomeric- and total-alpha-synuclein in premotor, motor and dementia stages of Parkinson's disease. J Neurol 262:294–306
Fairfoul G, McGuire LI, Pal S, Ironside JW, Neumann J, Christie S, Joachim C, Esiri M, Evetts SG, Rolinski M, Baig F, Ruffmann C, Wade-Martins R, Hu MT, Parkkinen L, Green AJ (2016) Alpha-synuclein RT-QuIC in the CSF of patients with alpha-synucleinopathies. Ann Clin Transl Neurol 3:812–818
Groveman BR, Orrù CD, Hughson AG, Raymond LD, Zanusso G, Ghetti B, Campbell KJ, Safar J, Galasko D, Caughey B (2018) Rapid and ultra-sensitive quantitation of disease-associated α-synuclein seeds in brain and cerebrospinal fluid by αSyn RT-QuIC. Acta Neuropathologica Communications 6
Gupta N, Shyamasundar S, Patnala R, Karthikeyan A, Arumugam TV, Ling EA, Dheen ST (2018) Recent progress in therapeutic strategies for microglia-mediated neuroinflammation in neuropathologies. Expert Opin Ther Targets 22:765–781
Hall S, Surova Y, Ohrfelt A, Zetterberg H, Lindqvist D, Hansson O (2015) CSF biomarkers and clinical progression of Parkinson disease. Neurology 84:57–63
Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT (2008) The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol 9:857–865
Hamilton RL (2000) Lewy bodies in Alzheimer's disease: a neuropathological review of 145 cases using alpha-synuclein immunohistochemistry. Brain Pathol (Zurich, Switzerland) 10:378–384
Holdorff B (2006) Fritz Heinrich Lewy (1885-1950). J Neurol 253:677–678
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181–184
Kondru N, Manne S, Greenlee J, West Greenlee H, Anantharam V, Halbur P, Kanthasamy A, Kanthasamy A (2017) Integrated Organotypic slice cultures and RT-QuIC (OSCAR) assay: implications for translational discovery in protein Misfolding diseases. Sci Rep 7:43155
Lee HJ, Suk JE, Bae EJ, Lee SJ (2008) Clearance and deposition of extracellular alpha-synuclein aggregates in microglia. Biochem Biophys Res Commun 372:423–428
Lema Tomé CM, Tyson T, Rey NL, Grathwohl S, Britschgi M, Brundin P (2013) Inflammation and α-Synuclein’s prion-like behavior in Parkinson's disease—is there a link? Mol Neurobiol 47:561–574
Maltsev AS, Ying J, Bax A (2012) Impact of N-terminal acetylation of alpha-synuclein on its random coil and lipid binding properties. Biochemistry 51:5004–5013
Manne S, Kondru N, Nichols T, Lehmkuhl A, Thomsen B, Main R, Halbur P, Dutta S, Kanthasamy AG (2017) Ante-mortem detection of chronic wasting disease in recto-anal mucosa-associated lymphoid tissues from elk (Cervus elaphus nelsoni) using real-time quaking-induced conversion (RT-QuIC) assay: a blinded collaborative study. Prion 11:415–430
Marti MJ, Tolosa E, Campdelacreu J (2003) Clinical overview of the synucleinopathies. Mov Disord 18(Suppl 6):S21–S27
Montine TJ (2012) National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach 123:1–11
Moore SJ, West Greenlee MH, Kondru N, Manne S, Smith JD, Kunkle RA, Kanthasamy A, Greenlee JJ (2017) Experimental transmission of the chronic wasting disease agent to swine after Oral or intracranial inoculation. J Virol 91
Mori H, Oda M, Komori T, Arai N, Takanashi M, Mizutani T, Hirai S, Mizuno Y (2002) Lewy bodies in progressive supranuclear palsy. Acta Neuropathol 104:273–278
Ohrfelt A, Grognet P, Andreasen N, Wallin A, Vanmechelen E, Blennow K, Zetterberg H (2009) Cerebrospinal fluid alpha-synuclein in neurodegenerative disorders-a marker of synapse loss? Neurosci Lett 450:332–335
Polinski NK, Volpicelli-Daley LA, Sortwell CE, Luk KC, Cremades N, Gottler LM, Froula J, Duffy MF, Lee VMY, Martinez TN, Dave KD (2018) Best practices for generating and using alpha-Synuclein pre-formed fibrils to model Parkinson's disease in rodents. J Parkinsons Dis 8:303–322
Rosenthal LS, Drake D, Alcalay RN, Babcock D, Bowman FDB, Chen-Plotkin A, Dawson TM, Dewey RB Jr, German DC, Huang X, Landin B, McAuliffe M, Petyuk VA, Scherzer CR, Hillaire-Clarke CS, Sieber BA, Sutherland M, Tarn C, West A, Vaillancourt D, Zhang J, Gwinn K, on behalf of the PDBP consortium (2016) The NINDS Parkinson's disease biomarkers program. Mov Disord 31:915–923
Sano K, Atarashi R, Satoh K, Ishibashi D, Nakagaki T, Iwasaki Y, Yoshida M, Murayama S, Mishima K, Nishida N (2018) Prion-like seeding of misfolded alpha-Synuclein in the brains of dementia with Lewy body patients in RT-QUIC. Mol Neurobiol 55:3916–3930
Spillantini MG, Divane A, Goedert M (1995) Assignment of human alpha-synuclein (SNCA) and beta-synuclein (SNCB) genes to chromosomes 4q21 and 5q35. Genomics 27:379–381
Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M (1998) α-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proc Natl Acad Sci U S A 95:6469–6473
Stone DK, Reynolds AD, Mosley RL, Gendelman HE (2009) Innate and adaptive immunity for the pathobiology of Parkinson's disease. Antioxid Redox Signal 11:2151–2166
Tokuda T, Qureshi MM, Ardah MT, Varghese S, Shehab SA, Kasai T, Ishigami N, Tamaoka A, Nakagawa M, El-Agnaf OM (2010) Detection of elevated levels of alpha-synuclein oligomers in CSF from patients with Parkinson disease. Neurology 75:1766–1772
Tu PH, Galvin JE, Baba M, Giasson B, Tomita T, Leight S, Nakajo S, Iwatsubo T, Trojanowski JQ, Lee VM (1998) Glial cytoplasmic inclusions in white matter oligodendrocytes of multiple system atrophy brains contain insoluble alpha-synuclein. Ann Neurol 44:415–422
Uchikado H, DelleDonne A, Ahmed Z, Dickson DW (2006) Lewy bodies in progressive supranuclear palsy represent an independent disease process. J Neuropathol Exp Neurol 65:387–395
Volpicelli-Daley LA, Luk KC, Lee VMY (2014) Addition of exogenous α-Synuclein pre-formed fibrils to primary neuronal cultures to seed recruitment of endogenous α-Synuclein to Lewy body and Lewy neurite-like aggregates. Nat Protoc 9:2135–2146
Wang Y et al (2012) Phosphorylated alpha-synuclein in Parkinson's disease. Sci Transl Med 4:121ra120
Waxman EA, Giasson BI (2011) Induction of intracellular tau aggregation is promoted by alpha-synuclein seeds and provides novel insights into the hyperphosphorylation of tau. J Neurosci 31:7604–7618
West Greenlee MH, Lind M, Kokemuller R, Mammadova N, Kondru N, Manne S, Smith J, Kanthasamy A, Greenlee J (2016) Temporal resolution of misfolded prion protein transport, accumulation, glial activation, and neuronal death in the retinas of mice inoculated with scrapie. Am J Pathol 186:2302–2309
Williams GP, Schonhoff AM, Jurkuvenaite A, Thome AD, Standaert DG, Harms AS (2018) Targeting of the class II transactivator attenuates inflammation and neurodegeneration in an alpha-synuclein model of Parkinson's disease. J Neuroinflammation 15:244
Acknowledgments
We thank Gary Zenitsky for proofreading the manuscript and Griffin Clabaugh for technical assistance. We would like to thank Kayla Guthals for assistance with the figures. We also are indebted to Dr. Julien Roche, Iowa State University, for providing us with human WT αSyn-expressing plasmid, Dr. Douglas T. Golenbock, U Mass, for the mouse microglial cell line, Alzheimer’s disease Center at UC Davis for supplying AD, DLB and control samples (funded by NIH/NIA P30 AG10129), and the University of Miami Brain Endowment Bank of the NIH Neurobiobank for providing PD and control samples. The Lloyd and Armbrust endowments to AGK and Salisbury endowment to AK are also acknowledged. This study was supported in part by the following sources: National Institutes of Health grants ES026892 and NS100090 to AGK, and NS088206 to AK, as well as the Presidential Interdisciplinary Research Initiative for the Big Data Brain Research from Iowa State University. The financial support for human CSF and brain samples received by XH include NS060722 and NS082151 to the Hershey Medical Center Clinical Research Center (National Center for Research Resources, Grant UL1 RR033184 that is now at the National Center for Advancing Translational Sciences, Grant UL1 TR000127), National Center for Advancing Translational Sciences (TL1 TR002016), the PA Department of Health Tobacco CURE Funds (XH), the Translational Brain Research Center, the Michael J. Fox Foundation for Parkinson’s Research, Alzheimer’s Association, Alzheimer’s Research UK, and the Weston Brain Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
A.G.K. and V.A. have an equity interest in PK Biosciences Corporation located in Ames, IA. The terms of this arrangement have been reviewed and approved by Iowa State University in accordance with its conflict of interest policies. Other authors declare no actual or potential competing financial interests.
Ethics Approval
CSF samples were collected as part of the NINDS Parkinson’s Disease Biomarker Program (PDBP) according to guidelines (Rosenthal et al. 2016) for the lumbar puncture. The protocol was reviewed and approved by the Penn State Hershey Internal Review Board and informed written consent was obtained from all subjects. All brain tissues of human subjects were from the University of Miami Brain Endowment Bank and the UC Davis Alzheimer’s Disease Center.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author Contributions
Conceptualization, S.M., N.K., and A.G.K.; Experiment design and performance, S.M. and N.K.; microglial uptake of αSyn, M.H., N.K., and S.M.; H.J. and V.A. assisted in the preparation of the manuscript; Clinical specimen acquisition, X.H., and M.L.; Funding Acquisition, A.G.K., A.K.; Supervision, A.G.K.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 129 kb)
Rights and permissions
About this article
Cite this article
Manne, S., Kondru, N., Hepker, M. et al. Ultrasensitive Detection of Aggregated α-Synuclein in Glial Cells, Human Cerebrospinal Fluid, and Brain Tissue Using the RT-QuIC Assay: New High-Throughput Neuroimmune Biomarker Assay for Parkinsonian Disorders. J Neuroimmune Pharmacol 14, 423–435 (2019). https://doi.org/10.1007/s11481-019-09835-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-019-09835-4