Abstract
There is growing evidence that Zika virus (ZIKV) infection is linked with activation of Guillan-Barré syndrome (GBS) in adults infected with the virus and microcephaly in infants following maternal infection. With the recent outpour in publications by numerous research labs, the association between microcephaly in newborns and ZIKV has become very apparent in which large numbers of viral particles were found in the central nervous tissue of an electively aborted microcephalic ZIKV-infected fetus. However, the underlying related mechanisms remain poorly understood. Thus, development of ZIKV-infected animal models are urgently required. The need to develop drugs and vaccines of high efficacy along with efficient diagnostic tools for ZIKV treatment and management raised the demand for a very selective animal model for exploring ZIKV pathogenesis and related mechanisms. In this review, we describe recent advances in animal models developed for studying ZIKV pathogenesis and evaluating potential interventions against human infection, including during pregnancy. The current research directions and the scientific challenges ahead in developing effective vaccines and therapeutics are also discussed.
Similar content being viewed by others
ZIKV Epidemiology and Transmission
Zika virus (ZIKV) is an emerging mosquito-borne virus (arbovirus) belonging to the genus Flavivirus and the family Flaviviridae, initially identified in the Zika Forest of Uganda in 1947 in rhesus monkeys and later in humans in 1952 (Hayes 2009). Additional members of the Flaviviridae family (Table 1) include several globally relevant human pathogens such as Yellow fever virus (YFV), Dengue fever virus (DENV), West Nile virus (WNV), Japanese encephalitis virus (JEV), and Tick-borne encephalitis virus (TBEV) (Unni et al. 2011; Lazear et al. 2016). According to comparative genomic and phylogenetic analyses, there are several homologue strains of ZIKV (Fig. 1 ) including; (i) the African lineage (Zika virus Uganda), (ii) the Asian lineage which has recently emerged in the Pacific (Zika virus Senegal) and (iii) the South American lineage (Zika virus Brazil) (Unni et al. 2011; Lazear et al. 2016; Faye et al. 2014). Since the early part of the twentieth century, mosquitos of the Aedes genus have been implicated in the spread of ZIKV, and are of particular concern for many parts of the world owing to their broad distribution (Kraemer et al. 2015). Although ZIKV was first isolated from A. africanus in the Zika forest, located in the Entebbe Peninsula of Uganda (Dick et al. 1952; Weinbren and Williams 1958), the first report of A. aegypti as a ZIKV vector came several years later when the virus was isolated from this species in the Malay Peninsula (Marchette et al. 1969). The following year, A. luteocephalus was also identified as a vector of ZIKV (Lee and Moore 1972), and although it was long suspected to be a vector, A. albopictus was only recently identified to be capable of carrying the virus (Wong et al. 2013). Among the genuses, A. aegypti and A. albopictus are of significant concern for the spread of ZIKV to humans. Female A. albopictus bite during daytime hours and both aegypti and albopictus species are attracted to warm, urban environments (Powell and Tabachnick 2013) with areas of standing water which boost their wider geographical range (Delatte et al. 2008).
a Comparative genomic analysis of ZIKV using BLAST Atlas. Genome shared identity between each strain and the reference genome are shown as percentages. Brazilian ZIKV (green circle) isolated from amniotic fluid of a patient whose fetus was diagnosed with microcephaly, yielded 97–100% genomic identity with the reference genome sequence KJ776791.1 isolated in French Polynesia (innermost black circle). The genome sequence NC_012532.1 isolated from Africa (Zika virus Uganda), indicated by the blue circle, and the genome sequence KF383118.1 (Zika virus Senegal), indicated by the red circle, yielded 87–90% identity. 10,793 bases were sequenced and the proportion of GC content in the Brazilian Zika virus was 51.2%. BLAST = basic local alignment search tool (Figure reprinted Ref. (Calvet et al. 2016), Copyright The Lancet). b ZIKV Phylogenetic Classification. Strains within the ZIKV branches are identified with the GenBank accession number, year of isolation, and country of origin (except those identified in 2015–2016). The evolutionary history was inferred by means of the neighbor-joining method under a GTR + G + I substitution model. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (2000 replicates) is shown next to the branches. Phylogenetic analysis of a complete ZIKV genome sequence (10,808 nucelotides) recovered from fetal brain tissue in Bahia, Brazil (KU527068) showed highest identity (99.7%) with the ZIKV strain isolated from a patient from French Polynesia in 2013 (KJ776791) and ZIKV detected in Sao Paolo, Brazil, in 2015 (KU321639). 98.3 and 98% identity were detected with JN860885, a strain isolated in Cambodia in 2010 and EU545988, a strain isolated from the outbreak in Micronesia in 2007, respectively. The genetic distance in nucleotide substitutions per site is indicated by the 0.01 scale bar. (Figure reprinted Ref. (Mlakar et al. 2016 ), Copyright The New England Journal of Medicine)
The most common mode of transmission of arboviruses is horizontal transmission where mosquitos take a viremic blood meal and then inject infectious saliva into a host during blood feeding (Kenney and Brault 2014). ZIKV seems to have modified itself to humans and can maintain a large population in a mosquito-human-mosquito transmission cycle where non-human reservoirs are not mandatory for transmission. During a mosquito bite, the virus is delivered into extravascular spaces in the epidermal and dermal areas of the skin of a vertebrate host. The initial cycle occurs at the site of the bite after the virus gains access to resident and migratory cells in the skin. The mosquito salivary factor, delivered along with the virus, potentiates its capacity to replicate leading to an enhanced viremia which, in turn, contributes to an acute viral pathology (Briant et al. 2014). Keratinocytes, dermal fibroblasts, and dendritic cells in the skin are the initial targets of ZIKV infection (Hamel et al. 2015). Several cell surface molecules including TAM receptor AXL, DC- SIGN, Tyro-3, and TIM-1 have been demonstrated to support viral entry into cells (Gatherer and Kohl 2016). Viral replication is further supported by the formation of autophagosomes containing viral particles inside skin fibroblasts (Hamel et al. 2015). After a period of replication in the primary inoculation site, the virus is disseminated by dendritic cells via the lymphatics and blood stream (Hamel et al. 2015). Viremia develops 1 to 5 days later and the virus is usually cleared from the circulation within two weeks of infection (Bell et al. 1971). Replication of the virus leads to the activation of an antiviral innate immune response by the host, which involves the production of both type I and II interferons in infected cells (Hamel et al. 2015).
Non-vector arbovirus transmission routes include transmission from mother to fetus (in utero), blood transfusion, saliva, urine and bone marrow and organ transplantation (Kuno 2001; Oster 2016; Gao et al. 2016). Furthermore, ZIKV is the only arbovirus isolated from human semen which supports the hypothesis that ZIKV can be transmitted by sexual intercourse (Foy et al. 2011; Musso et al. 2015; Venturi et al. 2016). The first case of sexually transmitted ZIKV was reported in 2011, between an infected male who was exposed to the virus while traveling to a ZIKV-infected area and later transmitting the virus to his female partner (Foy et al. 2011). Another case reported transmission between a male traveler infected with ZIKV who transmitted the virus through anal intercourse with his male partner. Although both men had exhibited Zika symptoms, the male traveler had a confirmed infection of ZIKV and dengue, while his partner appeared to have only contracted ZIKV. Semen samples collected 17 and 24 days post-onset of the illness showed that only the traveler had detectable levels of ZIKV present in semen while his partner did not (Deckard et al. 2016). Other studies have detected ZIKV in semen supporting the notion of sexual transmission (Musso et al. 2015). The virus has also been detected in breast milk, although breastfeeding-associated transmission has not yet been reported (Boadle 2016; Cavalcanti et al. 2017). Additional aspects associated with the emergence of arboviruses include climate change and an increase in the geographical distribution range of mosquito vectors as well as their increased capacity to adapt to new reservoirs or amplification hosts. Increasing populations and urbanization, inefficient mosquito control, and extensive travel to affected areas are all factors which play a major role in intensifying the disease distribution (Kilpatrick and Randolph 2012).
ZIKV Pathogenesis and Cases of Microcephaly
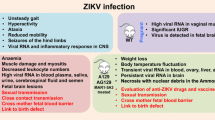
Although there is no cure for ZIKV, in most cases, only 20% of patients will present clinical symptoms which will usually resolve within 2 to 7 days, and rarely requires hospitalization (Petersen et al. 2016; Shuaib et al. 2016). The clinical manifestations of ZIKV infection illustrated in Fig. 2 resemble that of other mosquito-transmitted diseases like Chikungunya (CHIKV) and Dengue fever virus (DENV), but with substantially lower mortality than DENV (Fox 2015). In additon, ZIKV-related symptoms are usually milder and of shorter duration, and they present with a mild fever of about 98.9 °F (37.2 °C) and a combination of one or more of the following symptoms: conjunctivitis, macular or papular rash, arthralgia, myalgia, headache, retro-orbital pain, edema, vomiting, and weakness.
ZIKV contains a single-stranded, positive-sense RNA of about 10,000 nucleotides (nt), encompassing a coding region of 10 genes (Pierson and Kielian 2013; Marano et al. 2016). Like other Flaviviruses, the genomic RNA contains a single open reading frame (ORF) that encodes for a polypeptide which is further processed post-translationally by both host and viral proteases into 3 structural proteins (Fig. 3a–b). The polyproteins are organized from 5′ to 3′ with both ends of the ORF covered by a 100 nt untranslated region (UTR). The viral structural proteins consisting of the capsid (C), pre-membrane (prM), and envelope (E), are located towards the 5′ end, while the seven non-structural (NS) proteins consisting of NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5 are found at the 3′ portion (Chambers et al. 1990; Kuno and Chang 2007; Hulo et al. 2011; Cunha et al. 2016). The C protein interacts with viral RNA to form the nucleo-capsid, prM impedes premature fusion with host membranes, and the E protein functions in cellular attachment, fusion and entry (Mukhopadhyay et al. 2005). The NS proteins are involved in attenuating host anti-viral responses while also controlling viral transcription and replication (Diamond and Pierson 2015; Pierson and Kielian 2013; Suthar et al. 2013). Flavivirus genomic RNA are high in their purine content and have low CG and UA doublet occurrences; this fact needs to be studied more to understand its significance (Chambers et al. 1990).
Active ZIKV Transmission and Potential Neurological Disorder in Human. a Illustrates a 3D imaging of ZIKV and (b) shows the genomic sequence and encoded proteins. c Severe macular neuroretinal atrophy in an infant with microcephaly [Figure reprinted Ref. (Ventura et al. 2016), Copyright Elsevier-2016]. d Brain MRI from a 3-month-old infant born with microcephaly. The mother acquired ZIKV at 12 weeks gestation and ultrasound carried out at the end of gestation showed reduced head circumference. The child was born prematurely (34 weeks) and the images illustrate (a) calcifications; (b) cortical development malformation; (c) posterior fossa malformation and redundant skin [Figure reprinted Ref. (Araujo et al. 2016 ), Copyright Oxford University Press]
Microcephaly is a neurological disorder associated with abnormal eye and brain development in fetuses (Fig. 3c–d ). Accordingly, more than 850 babies were born with brain damage and other clinical signs of microcephaly to ZIKV-infected mothers in Brazil (Sun 2016). Association between ZIKV infection and microcephaly via vertical transmission of virus from pregnant mother to her fetus was illustrated based on an increased incidence of microcephaly possibly pertaining to ZIKV outbreak, presence of ZIKV in microcephalic fetal brain as well as the presence of viral RNA in the amniotic fluid of pregnant women (Calvet et al. 2016; Driggers et al. 2016; Marrs et al. 2016; Mlakar et al. 2016). ZIKV detected in the brain tissue of microcephaly-affected fetuses and infants further supports a causal relationship of the virus to the neurological disorder (Rasmussen et al. 2016). In one particular case, a pregnant woman in her 11th week of gestation tested positive for ZIKV IgM antibodies four weeks after returning from a week-long trip to Mexico, Guatemala, and Belize (Brasil et al. 2016). Based on MRI and ultrasonography imaging at 19–20 weeks of gestation, the fetus was found to have multiple brain anomalies including calcification, cortical development malformation, posterior fossa malformation and redundant skin (Fig. 3d ). Microcephaly was not diagnosed by the end of the 21-week gestation period. The circumference of the head was reported to be decreased from 47th percentile (16 weeks) to 24th percentile (20 weeks), which indicated that there would have been high chances of microcephaly development if the pregnancy had continued (Araujo et al. 2016). In the case of a 20-year-old Brazilian woman, abnormal fetal development was detected during her 19th-week of gestation by ultrasound, although the woman had no symptoms of ZIKV. During the 26th and 30th weeks of gestation, the fetus was diagnosed with microcephaly and hydrops fetalis by ultrasounds and by 32 weeks the fetus was determined to be deceased. Tissue analysis confirmed detection of ZIKV in the amniotic fluid and cerebral spinal fluid but not in the placenta, eyes, heart, lungs, or liver (Sarno et al. 2016). In the continental U.S. and U.S. territories there are about 3808 and 84 cases, respectively, of travel-related cases of Zika and 128 locally-spread cases in the continental U.S. and 25,871 cases in the U.S. territories (CDC). Accordingly, the state of Florida has the most travel-related (708) and local (128) cases, second only to Puerto Rico, with 75 travel-related and 25,355 locally-acquired cases (CDC). The first Zika-related microcephaly birth in Florida was delivered by a woman of Haitian nationalization who came to Florida to give birth, while she was infected with ZIKV in Haiti. There have previously been other Zika-related microcephaly births in the U.S., including one in Hawaii, one in New Jersey, two in California and one case in Texas. In every case, the baby’s mother had traveled to either the Caribbean or South America and contracted the virus overseas. In total, seven babies have been born in the U.S. with birth defects related to Zika as of June 30, 2016, according to the CDC. Zika-related birth defects were also linked to pregnancy losses, which includes miscarriages and abortions (CDC).
Neurological Aspects of ZIKV Pathology
The primary concern of ZIKV infection is its association with neurodevelopmental complications like microcephaly. The cause for the characteristic abnormally small heads and underdeveloped brains in children and infants can be attributed to several factors including: (i) pathogenic viral infections (e.g., human cytomegalovirus, rubella virus, and varicella-zoster virus), (ii) exposure to environmental toxins (e.g., drugs or alcohol), and (iii) genetic mutations (Bell et al. 1971; Mochida and Walsh 2001; Mendola et al. 2002; Mochida 2009, de Paula Freitas et al. 2016), resulting in deficiencies in the number of neurons (Bell et al. 1971; Mendola et al. 2002). More than 40 years ago, ZIKV tropism for brain was shown with viral transmigration across the blood-brain barrier infecting both neurons and glial cells in mouse brain (Bell et al. 1971). Recent studies have shown that the African and Asian ZIKV strain MR 766 is capable of infecting human neural precursor cells derived from human induced pluripotent stem cells (hiPSCs) (Tang et al. 2016). This infection induced cell death and deregulation of the cell-cycle progression of hiPSCs, and reduced their viability and growth as neurospheres and brain organoids (Garcez et al. 2016; Qian et al. 2016; Tang et al. 2016), although no association was identified with microcephaly. Figure 4a–b shows a graphical representation of how ZIKV infects human cortical neural progenitors and consequently attenuates growth. With the high incidence of up to a 20-fold increase in the children born with microcephaly in Brazil, public health officials raised concerns of a link between ZIKV and microcephaly with appropriate health-risk warnings issued to pregnant women (Pylro et al. 2016; PAHO/WHO 2015). Although the CDC had initially cautioned that Flaviviruses are not known to cause microcephaly and that any possible association remains under investigation (Schuler-Faccini et al. 2015), numerous studies have associated ZIKV infections with cases of meningoencephalitis and acute myelitis (Carteaux et al. 2016; Mécharles et al. 2016). While much remains to be determined about the mechanisms by which ZIKV mediates microcephaly and other birth defects, different types of intracytoplasmic inclusion bodies collectively known as “virus factories” were shown to be produced by ZIKV infection in mouse brain cells. The virus factories or inclusion bodies were found primarily to originate from the endoplasmic reticulum, nucleus, and mitochondria (Bell et al. 1971). Centrosomes and autophagy hijacking have been implicated in ZIKV neural pathogenesis and microcephaly development (Thornton and Woods 2009; Marthiens et al. 2013). ZIKV can prevent completion of the autophagy process, providing a perfect environment for the making of “viral factories” to increase viral replication and amplification (Tetro 2016). Autophagy is a lysosomal degradative process that can be used by the host to combat viral infection (Deretic et al. 2013), and recent studies have demonstrated an important role in the induction of this pathway and ZIKV replication in human skin (Hamel et al. 2015) and brain cells (Liang et al. 2016). Some cellular proteins like UVRAG (ultraviolet irradiation resistance-associated gene) and Beclin-1 have a role in both autophagy and centrosome stability in dividing cells. Increased numbers of centrosomes, the cellular organelles involved in cell division as well as vesicle migration and trafficking within cells, are shown to be associated with delayed mitosis, premature neural differentiation and a higher rate of apoptosis, all of which contribute to microcephaly in mice (Tetro 2016). Owing to this fact, the sequels of ZIKV infection may be broader than what is considered because the roles of centrosomes and autophagy may not be limited to neural development. Complications with ZIKV can also include the association with Guillain-Barré syndrome (GBS), as was confirmed very recently by magnetic resonance imaging (MRI) findings (Fontes et al. 2016). GBS is an autoimmune neuropathy that can result in muscle weakness, paralysis, and death as a consequence of the immune-mediated demyelination of peripheral nervous system neurons. A temporal and geographic relationship between GBS and ZIKV infection in adults was established during the 2013–2014 outbreak in French Polynesia, although the complete association and pathophysiology are still unclear (Hancock et al. 2014). Fontes et al. (2016) reported that ZIKV-associated GBS was characterized by demyelination, ischemia, inflammation, and breakdown of the blood-brain barrier. Cao-Lormeau et al. (2014, 2016) observed anti-ZIKV virus IgM or IgG in 98% of the patients diagnosed with this syndrome during the outbreak. Reported cases of widespread inflammatory demyelinating disorder similar to GBS were also temporally associated with ZIKV infection in most parts of South and Central America, including Brazil, El Salvador, Colombia, and Venezuela (ECDC First and Second update 2016a, b). Patients with presumable ZIKV-associated GBS had atypically low levels of the anti-ganglioside antibodies associated with the most prevalent type of the syndrome in Asia (acute motor axonal neuropathy), compared to patients with GBS of other etiologies, such as influenza, Campylobacter jejuni, Cytomegalovirus and Epstein-Barr virus infections, suggesting that ZIKV may induce GBS by different mechanisms which remain to be elucidated (Cao-Lormeau et al. 2016). Until now, ZIKV-related GBS in adults seems to be transient in duration, where the majority of patients tend to recover almost completely (Weaver et al. 2016). One report from Brazil refers to 6 patients, aged 2–5 years, with neurologic syndromes (4 with GBS and 2 with acute disseminated encephalomyelitis) after laboratory-confirmed ZIKV infection, however, no further data on the association of ZIKV infection and GBS in infants are available (CDC).
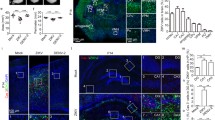
Illustration of Brain Cells Infected with ZIKV and Cell Cycle Arrest (a) ZIKV infects human cortical neural progenitors and attenuates their growth [Figure reprinted Ref. (Tang et al. 2016 ), Copyright Elsevier]. b Disruption of Phospho-TANK-binding kinase (TBK)1 localization and mitosis in ZIKV-infected human neuroepithelial stem cells (NES) and radial glial cells (RGC) [Figure reprinted Ref. (Onorati et al. 2016 ), Copyright Elsevier]
ZIKV and Related Animal Models
Animal models are essential for understanding the transmission and infection of pathogens, as well as for evaluating candidate vaccines and therapeutics. With the advent of animal research, we have a better understanding of the ZIKV infection mechanism and are able to answer several questions including: (i) whether ZIKV can be transmitted in utero by pregnant mothers and how does the virus affect the fetus and (ii) is there any direct association between ZIKV and microcephaly. The ZIKV epidemic and the congenital abnormalities associated with infection have brought an urgent need for developing in vivo models, including: (i) chicken embryo, (ii) mouse (iii) monkey and potentially, (iv) hamster models. In a recent mini-review article by Morrison and Diamond, they elegantly reviewed the various animal models available to study ZIKV pathogenesis, including their utility and limitations (Morrison and Diamond 2017). Tables 2, 3, 4 and 5 compare the different species of animal used as potential models in ZIKV infection and their utility to understand disease pathogenesis. One animal model that resembles the human fetal neurodevelopment is the ovo development of the chicken embryo (Goodfellow et al. 2016). Using chick embryos infected with a Mexican strain of ZIKV, one group showed dose dependent embryonic lethality when embryos were infected with high doses of ZIKV, and sustained infectivity with no chick mortality when embryos were infected at low viral doses and during later stages of chick development. Chick embryos developed central nervous system structural malformation and microcephaly-like pathology as determined by MRI. Based on this study, the chicken embryo model offers a comparative animal model that can provide key insight into ZIKV pathogenesis (Goodfellow et al. 2016; Morrison and Diamond 2017). The mouse model is predominantly based on immunocompromised animals lacking the receptor for type I interferon (IFN α/β) (A129 mice) or types I and II IFN (IFN α/β/γ) (AG129 mice) (Ifnar1−/−). These animals are highly susceptible to ZIKV infection and sustain infection with high viral loads in the brain and spinal cord, consistent with the severe neurological manifestation of ZIKV in humans, and high viral loads in the testes, relevant to the sexual transmission observed with ZIKV in humans (Aliota et al. 2016a, b; Dowall et al. 2016; Rossi et al. 2016).
Immunocompromised mice including A129 mice, Ifnar1−/−, or mice deficient in Irf-3/5/7−/− infected with the Asian (H/PF/2013), American (Brazil Paraiba_2015) or African (MR766) strain of ZIKV were permissive to ZIKV infection and vulnerable to ZIKV-related pathology and mortality. Similar effects were observed in normal C57BL/6 mice injected with anti-IFNAR1 antibodies (that suppress expression of human type 1 interferon) before and after infection with the African (strain Dakar 1984) strain of ZIKV. Furthermore, these studies showed that severity of ZIKV infection is age dependent, as older mice (11 week-old) are less susceptible to infection than younger mice (3–5 week-old) (Morrison and Diamond 2017). In utero transmission of ZIKV with evidence of microcephaly and growth restriction in mouse brain has been elegantly illustrated (Cugola et al. 2016; Goodfellow et al. 2016; Lazear et al. 2016; Miner et al. 2016). Miner et al. (2016) developed two mouse models that support ZIKV replication as well as trans-placental transmission in pregnant dams. In the first model they used ZIKV-infected immunocompromised pregnant Ifnar1 −/− dams that resulted in fetal demise, and in the second model they used a less severe model of ZIKV pathogeneses of normal (immunocompetent) pregnant dams that were given anti-IFNAR antibody prior to and during infection which did not cause fetal death. The pregnant dams were infected with ZIKV at embryonic (E) gestation period E5-7 which is around the period (E-10) when neurogenesis begins (Finlay and Darlington 1995). Both mouse model studies suggest that ZIKV can infect and damage the placenta, and cause microcephaly and growth restriction. The same group also showed that ZIKV can cause eye and brain infection in adult mice independently of the AXL receptor. Infection results in conjunctivitis, panuveitis, and infection of the cornea, iris and optic nerve in the retina (Miner et al. 2016; Morrison and Diamond 2017). In addition to the eye, ZIKV RNA was detected in the male reproductive tract including sperm cells, sertoli, testis (seminiferous tubules) and epididymis of normal C57BL/6 mice (treated with anti-IFNAR1) and Ifnar1−/− mice infected with ZIKV (strain Dakar 41,519) (Morrison and Diamond 2017). Of note is that levels of ZIKV RNA detected in the uterus of infected C57BL/6 (Ifnar1−/−) mice were surprisingly low in vaginal fluids, which might indicate a lower possibility for female-to-male sexual transmission, despite posing substantial risk to pregnancy. Moreover, C57BL/6 (Ifnar1−/−) mice intravaginal inoculation of ZIKV also showed viral presence in the vagina, uterus and ovary (Morrison and Diamond 2017). In a study by Cugola et al. (2016), they used normal SJL and C57BL/6 pregnant dams infected with a Brazilian ZIKV strain. Pups born from the SJL ZIKV-infected pregnant females displayed evidence of whole-body growth delay and intra-uterine growth restriction, while the brains showed evidence of cortical malformations with reduced cell number and cortical layer thickness, which are signs associated with microcephaly in humans (Li et al. 2016a, b; Cugola et al. 2016). Other studies also support the findings of vertical transmission of ZIKV infection in pregnant mice affecting fetal brain development (Wu et al. 2016). Although the animal model showed placental and fetal brain damage, and supports in vitro findings that the virus affects neuronal cells, this model did not show fetal development of microcephaly (Lazear et al. 2016). Other animal models bypassed the placenta and used intracerebral inoculation methods to infect fetal brains directly. In the study by Li et al., they used timed pregnant mice and embryonic mouse brains injected with the Asian ZIKV strain SZ01 in the lateral ventricle at gestation period E13.5. Embryos inspected at E16.5 or E18.5 of gestation showed increased immune responses and apoptosis in ZIKV-infected neurons which, according to the authors, could be a possible association of ZIKV with the development of microcephaly (Li et al. 2016b). Injection with the Mexican isolate (MEX 1-44) into the lateral ventricles of E14.5 embryo brains in timed pregnant C57BL/6J or 129S1/SvImJ mice (Shao et al. 2016), confirmed prior findings of brain abnormalities and growth restriction (Cugola et al. 2016; Miner et al. 2016), as well as major aspects of abnormal fetal brain connected with ZIKV in humans, including extensive neuronal cell death and loss, axonal rarefaction, leaky blood-brain-barrier (BBB), and gliosis (Driggers et al. 2016; Mlakar et al. 2016; Oliveira Melo et al. 2016), which have not been described in previous animal models (Table 3). Animal studies also suggest that neuronal cell death contributes significantly to the microcephaly associated with ZIKV, consistent with the selective neuronal vulnerability to ZIKV observed in humans (Driggers et al. 2016). Of note is that classical microcephaly is characterized by small head and brain size while the rest of the body size is relatively well developed (Thornton and Woods 2009; Nigg and Stearns 2011). However, the ZIKV-infected pups and embryos described in the animal studies showed a growth restriction with an overall smaller body size when compared to their mock-infected counterparts. The small head was in proportion with their small body size, and did not appear to have the characteristic smaller head size observed in microcephalic babies. Although the use of immunocompromised mice is not ideal for describing the pathogenesis of ZIKV, as mice lacking the ability to respond to interferon molecules have increased susceptibility to viral disease, these animal models appear to be well-suited to screen anti-viral compounds and test vaccine efficacy (Aliota et al. 2016a, b; Rossi et al. 2016).
Unlike mouse models which do not mimic key attributes of human genetics, infection and fetal development, nonhuman primates are uniquely suited as models in both infectious disease and obstetric research (Dudley et al. 2016). Using rhesus macaques as a nonhuman primate model, Dudley et al. (2016), demonstrated that both male and female rhesus macaques can succomb to ZIKV infection of Asian lineages. The infected macaques undergo rapid viral replication in the blood and display signs of immune activation and proliferation leading to the development of virus-specific T cells. ZIKV was also detected in urine and saliva of the macaques, and occasionally in the cerebral spinal fluid or vaginal secretions of the female macaques (Dudley et al. 2016). In a similar study by Osuna et al. (2016), they used two different ZIKV strains to infect two different strains of male and female macaques and evaluated the dynamics of ZIKV viremia, in cerebrospinal fluid and mucosal secretions of these macaque species. They also showed that rapid innate and adaptive immune responses limit viral replication in the blood and that these responses provide defense from future reinfection. Both studies showed that despite ZIKV-specific immunity, viral shedding continues persistently in anatomic tissues and fluids such as the male genital tract and oral mucosa which might harbor unabated reservoirs of ZIKV that, under certain conditions, could enable viral transmission over prolonged times to uninfected hosts. Of note, they found sustained high-level production of infectious virus in the male genital tract, highlighting the possibility of sexual transmission long after the resolution of symptomatic infection. Using a pregnant pigtail macaque model of ZIKV infection, Adams et al. (2016) showed fetal brain lesions within 10 days that evolved asymmetrically in the occipital–parietal lobes. Autopsy analysis from fetal brain showed ZIKV infection and substantial pathology to the central nervous system illustrated by hypoplasia of the cerebral white matter, gliosis in the periventricular white matter, and damage to the axonal and ependymal area. Collectively, the studies demonstrate that non-human primates are deemed more relevant than mouse models for studying ZIKV infection in gestation because macaques, unlike mice, most closely resemble human pregnancy in various key aspects, including placental barrier, gray/white matter ratios in the brain and period of neurodevelopment (Table 4). Findings from these studies not only highlight the need for the rapid development of vaccines and therapeutics against ZIKV but also illustrate that macaque models can be utilized for testing protective efficacy of novel vaccines and therapeutics against the ZIKV syndrome present from birth.
Other animal models such as hamster have been previously reported for both DENV and WNV infection, and could potentially serve as a choice animal model to study ZIKV transmission and pathogenesis as was recently reported by Chan et al. (2016). In this study hamster kidney cell lines (BHK21) showed susceptibility to ZIKV strains isolated from human and non-human primates (Table 5). Findings of efficient ZIKV replication in non-human cell lines suggest a role in the transmission of ZIKV. More importantly, this study illustrates that alternative animals such as hamsters should be evaluated as potential animal models for ZIKV infection, as interferon signaling (Ifnar1−/−, Irf3−/− C57BL/6) or receptor-deficient (IFN receptor type I and II AG129) mice may not be commonly available in some research laboratories (Chan et al. 2016). The advantage of hamsters over other rodents lies in their higher metabolism and physiological similarities to humans (Gao et al. 2014).
Overall, the animal models open doors to understanding ZIKV infection and its deteriorating effects during pregnancy, confirming its relationship to microcephaly, finding potential targets to test potential drugs, and vaccine development to successfully and quickly fight ZIKV epidemics. Consistent phenotypic outcomes from these different animal models would help to determine potential vaccines and drugs to treat ZIKV outbreaks.
Diagnostic Tools and Vaccination: A Potential Way to Prevent a ZIKV Epidemic
Observing clinical symptoms to diagnose ZIKV infection by itself is unreliable since there is high overlap with clinical manifestations produced by other Flaviviruses. Currently, the primary diagnostic tests for ZIKV are detection by reverse transcription-polymerase chain reaction (RT-PCR), which measures viral nucleic acids, and serological detection of IgM antibodies by IgM-captured enzyme-linked immunosorbent assay (MAC-ELISA). RT-PCR is the most accurate method to detect infection but it is not without its challenges; viral load is usually low, making it difficult to isolate enough from clinical samples, and it is most effective within one week of infection (Petersen et al. 2016). Limited research suggests that it is possible to isolate viral RNA from infected patients’ urine samples after one week of infection, increasing the detection window, however, further studies are required to confirm these initial observations (Gourinat et al. 2015). Serological tests serve as confirmation of RT-PCR when used as the initial test. They allow antibody detection after the viral load has decreased and is undetectable by RT-PCR, however, interpretation of these tests may be difficult due to cross-reactivity with other Flaviviruses (Lanciotti et al. 2008). Plaque-reduction neutralization testing (PRNT) can be performed as a follow-up to resolve false positives due to cross-reactivity. However, this test is time-consuming and requires delicate and expensive reagents, which makes this methodology impractical and in some cases unreliable (Roehrig et al. 2008). To overcome currently available laborious and expensive diagnostic tools, various groups of scientists are working on developing selective and cost effective diagnostic tools sensitive to picomolar concentrations of ZIKV with the help of biosensors (Kaushik et al. 2016; Pardee et al. 2016).
In modern medicine one of the most efficient interventions to control infectious epidemics is vaccines. More than 70 vaccines have been licensed to use and fight various diseases (Nabel 2013). There are currently no vaccines available for ZIKV and, of the different Flaviviruses, effective vaccines are available against Yellow fever virus, Japanese encephalitis virus, and West Nile virus (Ishikawa et al. 2014). This suggests that vaccine development against ZIKV is possible and could be the most effective approach to combat its rapidly spreading rate (Nah et al. 2015; Cohen 2016; Halstead and Russell 2016). The race for vaccine development against ZIKV started when health officials declared a connection between microcephaly and ZIKV infection. It led to the start of different pharmaceutical companies using different approaches (listed below) to make an effective vaccine against ZIKV (WHO 2016). Considering safety mandates, an inactivated virus vaccine meets with the most approval whereas the most effective may be a live attenuated form as it mimics the course of viral infection. One of the obstacles that may be faced during the trials is the lack of cases as the rate of infection may dwindle as was seen with the 2014 Ebola epidemic.
In hopes of developing a ZIKV vaccine, scientist have isolated a panel of human monoclonal antibodies from subjects with prior ZIKV infection (Sapparapu et al. 2016; Morrison and Diamond 2017). From the panel of antibodies, they found that ZIKV-117 targeted a unique quaternary epitope on the envelope (E) protein dimer–dimer interface and elicited the most inhibitory response in normal C57BL/6 mice (treated with anti-IFNAR-1) following infection with a lethal dose of ZIKV. Therapeutic efficacy of ZIKV-117 was also validated in pregnant mice, suggesting that neutralizing human monoclonal antibodies can protect against maternal–fetal viral transmission and attenuate ZIKV infection (Sapparapu et al. 2016; Morrison and Diamond 2017). The protective efficacy of (i) purified inactivated virus vaccine from ZIKV strain PRVABC59 and (ii) vector-based DNA vaccine expressing an optimized premembrane and envelope (prM-E) immunogen from adenovirus, was reported in non-human primates challenged with ZIKV strains from both Brazil (ZIKV-BR; Brazil/ZKV2015) and Puerto Rico (ZIKV-PR; PRVABC59). Around 95% of macaques that received the DNA plasmid prM-E-vaccine showed complete protection by developing antibodies when challenged with ZIKV-PR; PRVABC59. These data support the effectiveness of vaccine-based therapy against ZIKV challenge in rhesus monkeys and suggests future development of ZIKV vaccines for humans (Abbink et al. 2016; Dowd et al. 2016; Morrison and Diamond 2017).
An alternative to antibody-mediated or vector-based therapy is Sofosbuvir, a FDA-approved nucleotide polymerase inhibitor against the hepatitis C virus (Bullard-Feibelman et al. 2017; Morrison and Diamond 2017). Therapeutic efficacy of Sofosbuvir against ZIKV infection was observed in cell culture studies and in ZIKV-infected animals administered orally with the drug. These promising outcomes may lead to further evaluations of Sofosbuvir as a potential therapeutic agent against ZIKV infection in humans. In addition, Zmurko et al. (2016) reported that 7-deaza-2′-C-methyla-denosine (7DMA), an inhibitor of hepatitis C virus replication originally developed by Merck Laboratories, efficiently inhibits ZIKV infection and delays morbidity and mortality in the AG129 mouse model.
Since the Zika epidemic appears to be similar to the 2014 Ebola outbreak, it is necessary to have a system such as a global fund for vaccine development in place to avoid the absence of prevention after an outbreak in situations like Ebola and, currently, Zika (Plotkin et al. 2015; Plotkin 2016). This fund would reimburse companies who will develop a vaccine with proof of safety and efficacy in humans against epidemics. Moreover, the concept of a global fund was appreciated by government representatives, vaccine developers, and philanthropic organizations at the World Economic Forum in Davos, Switzerland held January 20–23, 2016. At the forum, the cost estimated for a single vaccine to pass clinical trial stages I, II and III approximated 100 million dollars. If this policy were to be successful, we would be able to react quickly to any outbreak anywhere in the world, but if not implemented we may face obstacles such as in the cases of Ebola and Zika in West Africa and Brazil, respectively (Pronker et al. 2013; Plotkin 2016). However, several questions need to be answered before scientists can continue with the development of a vaccine against ZIKV. For example, it is not clear whether an initial ZIKV infection would provide subsequent lifelong protection and if there is cross-protection from infection with other arboviruses. It is still not clear whether live attenuated vaccines or killed virus would be more efficient to boost the immune response. Furthermore, cause for concern relates to the notion that a ZIKV vaccine could increase the link between ZIKV infection and GBS (Cohen 2016; Maron 2016). There are close to 20 teams of biotech institutes and scientists globally that are involved in developing a ZIKV vaccine. A few of them are listed here with their endeavors: (i) Sanofi-Pasteur in Paris, France is recognized as one of the pioneering institutes that has been responsible for various vaccine developments and disbursement programs. Some of their vaccines include those for the Japanese encephalitis, dengue fever, and yellow fever viruses. This team is currently developing a live-attenuated vaccine for ZIKV. Live-attenuated forms retain many of the biological properties of the virus that make it immunologically recognizable, but do not have any of the virulence associated with the virus (Pasteur 2016); (ii) The Butantan Institute in Sao Paulo, Brazil is trying to develop a live-attenuated vaccine for ZIKV with the notion that live vaccines would be more efficient in boosting the immune response as compared to a killed virus vaccine. Another traditional approach used by Butantan is to develop ZIKV antibodies in horse by injecting ZIKV into horses and collecting horse serum. Their plan is to use these antibodies to neutralize ZIKV in infected patients (Cohen 2016); (iii) The US National Institute of Allergy and Infectious Diseases is developing a vaccine based upon their work already underway for the West Nile virus. The vaccine is DNA-based which is using the genetic material of ZIKV to illicit an immunological response (Fauci 2016); (iv) Bharat Biotech, located in India, has two potential ZIKV vaccine candidates initially developed against Chikungunya, one of which is an inactivated virus vaccine and the other a DNA recombinant vaccine. The inactivated virus vaccine is currently undergoing pre-clinical testing in animals (Hayden 2016); (v) A vaccine comprised of ZIKV genes in a plasmid is an approach now being used by Inovio Pharmaceuticals of Plymouth Meeting, Pennsylvania, USA. They have already started experiments in mice. However, this method is currently receiving little attention by vaccine developers due to its failure to generate a strong immune response to the DNA vaccines; (vi) The Jenner Institute in Oxford, U.K. is applying the same approach GlaxoSmithKline (GSK) used for the Ebola vaccine. The ZIKV vaccine is based on a simian adenoviral vector which expresses the antigens of the ZIKV required to elicit a cellular and humoral response without the aid of any adjuvant. Other vaccine players such as Hawaii Biotech, Honolulu and Protein Sciences of Meriden, Connecticut have also launched programs to work on ZIKV and are trying to produce ZIKV protein in insect cell lines (Cohen 2016).
Alternative Strategies to Control the ZIKV Epidemic
Wolbachia and RIDL (release of insects with dominant lethality) are also attractive tools to control a ZIKV outbreak. As a common reproductive parasite genus, Wolbachia infects arthropod species and has the potential to be an effective biocontrol agent by its ability to infect different host organs but having selectivity for eggs and testes. Wolbachia can spread across its host population via cytoplasmic incompatibility. A male mosquito infected with Wolbachia would lead to no hatching of eggs after mating with a wild-type uninfected female. Wolbachia infection in females may lead to infected eggs. Thus, over a short period it can spread rapidly in the wild population and can greatly reduce the host’s reproductive ability. Therefore, Wolbachia has gathered attention to be one of the mechanisms to control arboviruses and the ZIKV epidemic. A recent study showed A. aegypti giving refuge to Wolbachia, which was highly resistant to ZIKV infection, can impede transmission of the virus. Wolbachia-carrying mosquitos showed lower prevalence and intensity of ZIKV, with decreased disseminated infection and no evidence of infectious virus in their saliva (Dutra et al. 2016). Therefore, Wolbachia-harboring mosquitoes have the potential to be one of the alternatives to control a ZIKV outbreak, with RIDL being another. In RIDL, the male mosquito carries a lethal mutant gene. When a male with an RIDL gene mates with a wild-type female, it produces offspring which die before maturity (Aliota et al. 2016a, b; Dickens et al. 2016; Dutra et al. 2016). Taken together, these alternative tools represent a cost-effective mechanism that can be used to protect the community by reducing ZIKV transmission.
Concluding Remarks
ZIKV has quickly spread through South and Central America, at meanwhile, the viral infection is connected to a rise in cases of microcephalic babies preceded because of Zika infection in the pregnant mother. The co-existence of other Flaviviruses like DENV along with ZIKV increases the risk for complications in co-infected patients. Without any current efficient ZIKV vaccine, genetically modified mosquitoes could be one of the solutions. Using this method Oxitec Company claimed that they may be able to reduce the population of wild Aedes aegypti mosquitos across Brazil and Panama. Moreover, the WHO and the Pan American Health Organization (PAHO) have recommended and backed Oxitec solutions (FOX 2016; WHO 2016). Whether genetically modified mosquitoes could be used as a control measure is under the process of FDA oversight, which has asked for an opinion from the public before final approval to use for ZIKV control, specifically in the Florida Keys (Plotkin 2016; Fox 2016). The prominent tool in controlling ZIKV infection will be a vaccine, but, as it is known, such methods take prolonged time before they can be applied to humans. Furthermore, extensive information about the ZIKV life cycle and its interaction and communication with the human immune system and the central nervous system are necessary to understand the underlying mechanisms of ZIKV pathogenesis. As an initiative to improve new research and discoveries on ZIKV, the Governor of the state of Florida authorized $25 million in state funds to support Zika research. The Zika Research Grant Initiative provided grants for Zika research to pursue the following three goals including: (i) the development, testing, or delivery of a vaccine or other methods to prevent Zika infection; (ii) to develop innovative, cost-effective Zika testing methods or therapeutics; and (iii) to investigate health impacts of ZIKV on children and adults.
This review can serve as a guideline for readers to conduct research and to plan future directions towards the understanding of appropriate animal models to explore ZIKV pathogenesis, design of vaccines/drugs and optimization of diagnostic tools for the treatment and management of ZIKV-associated diseases.
References
Abbink P, Larocca RA, Rafael A, Bricault CA, Moseley ET, Boyd M, Kirilova M, Li Z, Nanayakkara O, Nityanandam R, Mercado NB (2016) Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science 353(6304):1129–1132
Adams WKM, Stencel-Baerenwald JE, Kapur RP, Studholme C, Boldenow E, Vornhagen J, Baldessari A, Dighe MK, Thiel J, Merillat S, Armistead B, Tisoncik-Go J, Green RR, Davis MA, Dewey EC, Fairgrieve MR, Gatenby JC, Richards T, Garden GA, Diamond MS, Juul SE, Grant RF, Kuller L, Shaw DW, Ogle J, Gough GM, Lee W, English C, Hevner RF, Dobyns WB, Gale M Jr, Rajagopal L (2016) Fetal brain lesions after subcutaneous inoculation of Zika virus in a pregnant nonhuman primate. Nat Med 22(11):1256–1259. doi:10.1038/nm.4193
Aliota MT, Caine EA, Walker EC, Larkin KE, Camacho E, Osorio JE (2016a) Characterization of lethal Zika virus infection in AG129 mice. PLoS Negl Trop Dis 10(5):e0004750. doi:10.1371/journal.pntd.0004750
Aliota MT, Peinado SA, Velez ID, Osorio JE (2016b) The wMel strain of Wolbachia reduces transmission of Zika virus by Aedes aegypti. Sci Rep 1(6):28792. doi:10.1038/srep28792
Araujo AQC, Silva MTT, Araujo APQC (2016) Zika virus-associated neurological disorders: a review. Brain 139:2122–2130
Barba-Spaeth G, Dejnirattisai W, Rouvinski A, Vaney MC, Medits I, Sharma A, Simon-Lorière E, Sakuntabhai A, Cao-Lormeau VM, Haouz A, England P (2016) Structural basis of potent Zika–dengue virus antibody cross-neutralization. Nature 536(7614):48–53
Bell TM, Field EJ, Narang HK (1971) Zika virus infection of the central nervous system of mice. Arch Gesamte Virusforsch 35(2):183–193
Boadle A (2016) Brazil reports Zika infection from blood transfusions http://www.reuters.com/article/us-health-zika-brazil-blood-idUSKCN0VD22N. Accessed 4 Feb 2007
Brasil P, Pereira JP, Gabaglia CR, Damasceno L, Wakimoto M, Nogueira RMR et al (2016) Zika virus infection in pregnant women in Rio de Janeiro - preliminary report. N Engl J Med. doi:10.1056/NEJMoa1602412
Briant L, Desprès P, Choumet V, Missé D (2014) Role of skin immune cells on the host susceptibility to mosquito-borne viruses. Virology 464-465:26–32. doi:10.1016/j.virol.2014.06.023
Bullard-Feibelman KM, Govero J, Zhu Z, Salazar V, Veselinovic M, Diamond MS, Geiss BJ (2017) The FDA-approved drug sofosbuvir inhibits Zika virus infection. Antivir Res 137:134–140. doi:10.1016/j.antiviral.2016.11.023
Calvet G, Aguiar RS, Melo AS, Sampaio SA, de Filippis I, Fabri A, Araujo ES, de Sequeira PC, de Mendonça MC, de Oliveira L, Tschoeke DA, Schrago CG, Thompson FL, Brasil P, Dos Santos FB, Nogueira RM, Tanuri A, de Filippis AM (2016) Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: a case study. Lancet Infect Dis 16(6):653–660. doi:10.1016/S1473-3099(16)00095-5
Cao-Lormeau VM, Blake A, Mons S, Lastère S, Roche C, Vanhomwegen J, Dub T, Baudouin L, Teissier A, Larre P, Vial AL, Decam C, Choumet V, Halstead SK, Willison HJ, Musset L, Manuguerra JC, Despres P, Fournier E, Mallet HP, Musso D, Fontanet A, Neil J, Ghawché F (2016) Guillain-Barré syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet 387:1531–1539
Cao-Lormeau VM, Roche C, Teissier A, Robin E, Berry AL, Mallet HP, Sall AA, Musso D (2014) Zika virus, French polynesia, South Pacific, 2013. Emerg Infect Dis 20(6):1085–1086. doi:10.3201/eid2006.140138
Carteaux G, Maquart M, Bedet A, Contou D, Brugières P, Fourati S, Cleret de Langavant L, de Broucker T, Brun-Buisson C, Leparc-Goffart I, Mekontso Dessap A (2016) Zika virus associated with meningoencephalitis. N Engl J Med 374(16):1595–1596. doi:10.1056/NEJMc1602964
Cavalcanti MG, Cabral-Castro MJ, Gonçalves JL, Santana LS, Pimenta ES, Peralta JM (2017) Zika virus shedding in human milk during lactation: an unlikely source of infection? Int J Infect Dis 30(57):70–72. doi:10.1016/j.ijid.2017.01.042
Centers for Disease Control and Prevention (CDC) - National Center for Emerging and Zoonotic Infectious Diseases (2016) http://www.cdc.gov/zika/index.html. Accessed 29 Sept 2016; http://www.cdc.gov/dengue/index.html. Accessed 19 Jan 2016; www.cdc.gov/yellowfever. Accessed 12 July 2016; http://www.cdc.gov/westnile/index.html Accessed 13 Sept 2016
Chambers TJ, Hahn CS, Galler R, Rice CM (1990) Flavivirus genome organization, expression, and replication. Annu Rev Microbiol 44:649–688. doi:10.1146/annurev.mi.44.100190.003245
Chan JF, Yip CC, Tsang JO, Tee KM, Cai JP, Chik KK, Zhu Z, Chan CC, Choi GK, Sridhar S, Zhang AJ (2016) Differential cell line susceptibility to the emerging Zika virus: implications for disease pathogenesis, non-vector-borne human transmission and animal reservoirs. Emerg Microbes Infect 5(8):e93. doi:10.1038/emi.2016.99
Cohen J (2016) The race for a Zika vaccine is on. Science 351(6273):543–544. doi:10.1126/science.351.6273.543
Cugola FR, Fernandes IR, Russo FB, Freitas BC, Dias JL, Guimarães KP, Benazzato C, Almeida N, Pignatari GC, Romero S, Polonio CM, Cunha I, Freitas CL, Brandão WN, Rossato C, Andrade DG, Faria Dde P, Garcez AT, Buchpigel CA, Braconi CT, Mendes E, Sall AA, Zanotto PM, Peron JP, Muotri AR, Beltrão-Braga PC (2016) The Brazilian Zika virus strain causes birth defects in experimental models. Nature 534(7606):267–271. doi:10.1038/nature18296
Cunha MS, Esposito DL, Rocco IM, Maeda AY, Vasami FG, Nogueira JS, de Souza RP, Suzuki A, Addas-Carvalho M, Barjas-Castro Mde L, Resende MR, Stucchi RS, Boin Ide F, Katz G, Angerami RN, da Fonseca BA (2016) First complete genome sequence of Zika virus (Flaviviridae, Flavivirus) from an autochthonous transmission in Brazil. Genome Announc 4(2):e00032–e00016. doi:10.1128/genomeA.00032-16
de Paula Freitas B, de Oliveira Dias JR, Prazeres J, Sacramento GA, Ko AI, Maia M, Belfort R Jr (2016) Ocular findings in infants with microcephaly associated with presumed zika virus congenital infection in salvador, brazil. JAMA Ophthalmol. doi:10.1001/jamaophthalmol.2016.0267
Deckard DT, Chung WM, Brooks JT, Smith JC, Woldai S, Hennessey M, Kwit N, Mead P (2016) Male-to-male sexual transmission of Zika virus—Texas, January 2016. MMWR Morb Mortal Wkly Rep. 65(14):372-374. doi: 10.15585/mmwr.mm6514a3.
Dejnirattisai W, Supasa P, Wongwiwat W, Rouvinski A, Barba-Spaeth G, Duangchinda T, Sakuntabhai A, Cao-Lormeau VM, Malasit P, Rey FA, Mongkolsapaya J (2016) Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat Immunol. doi:10.1038/ni.3515
Delatte H, Dehecq JS, Thiria J, Domerg C, Paupy C, Fontenille D (2008) Geographic distribution and developmental sites of Aedes albopictus (Diptera: Culicidae) during a Chikungunya epidemic event. Vector Borne Zoonotic Dis 8(1):25–34. doi:10.1089/vbz.2007.0649
Deretic V, Saitoh T, Akira S (2013) Autophagy in infection, inflammation and immunity. Nat Rev Immunol 13(10):722–737. doi:10.1038/nri3532
Diamond MS, Pierson TC (2015) Molecular insight into dengue virus pathogenesis and its implications for disease control. Cell 162(3):488–492. doi:10.1016/j.cell.2015.07.005
Dick GW, Kitchen SF, Haddow AJ (1952) Zika virus (I). Isolations and serological specificity. Trans R Soc Trop Med Hyg 46(5):509–520
Dickens BL, Yang J, Cook AR, Carrasco LR (2016) Time to empower RIDL and Wolbachia against Zika. Open Forum Infect Dis 3(2):ofw103. doi:10.1093/ofid/ofw103
Dowall SD, Graham VA, Rayner E, Atkinson B, Hall G, Watson RJ, Bosworth A, Bonney LC, Kitchen S, Hewson R (2016) A susceptible mouse model for Zika virus infection. PLoS Negl Trop Dis 10(5):e0004658. doi:10.1371/journal.pntd.0004658
Dowd KA, Ko SY, Morabito KM, Yang ES, Pelc RS, DeMaso CR, Castilho LR, Abbink P, Boyd M, Nityanandam R, Gordon DN (2016) Rapid development of a DNA vaccine for Zika virus. Science. aai9137. doi:10.1126/science.aai9137
Driggers RW, Ho CY, Korhonen EM, Kuivanen S, Jääskeläinen AJ, Smura T, Rosenberg A, Hill DA, DeBiasi RL, Vezina G, Timofeev J, Rodriguez FJ, Levanov L, Razak J, Iyengar P, Hennenfent A, Kennedy R, Lanciotti R, du Plessis A, Vapalahti O (2016) Zika virus infection with prolonged maternal viremia and fetal brain abnormalities. N Engl J Med 374(22):2142–2151. doi:10.1056/NEJMoa1601824
Dudley DM, Aliota MT, Mohr EL, Weiler AM, Lehrer-Brey G, Weisgrau KL, Mohns MS, Breitbach ME, Rasheed MN, Newman CM, Gellerup DD, Moncla LH, Post J, Schultz-Darken N, Schotzko ML, Hayes JM, Eudailey JA, Moody MA, Permar SR, O'Connor SL, Rakasz EG, Simmons HA, Capuano S, Golos TG, Osorio JE, Friedrich TC, O'Connor DH (2016) A rhesus macaque model of Asian-lineage Zika virus infection. Nat Commun 7:12204. doi:10.1038/ncomms12204
Dutra HL, Rocha MN, Dias FB, Mansur SB, Caragata EP, Moreira LA (2016) Wolbachia blocks currently circulating Zika virus isolates in Brazilian Aedes aegypti mosquitoes. Cell Host Microbe 19(6):771–774. doi:10.1016/j.chom.2016.04.021
European Centre for Disease Prevention and Control (2016a) Rapid risk assessment. Zika virus disease epidemic: First update Stockholm: ECDC http://ecdc.europa.eu/en/publications/_layouts/forms/Publication_DispForm.aspx?List=4f55ad51-4aed-4d32-b960-af70113dbb90&ID=1427
European Centre for Disease Prevention and Control (2016b) Rapid risk assessment. Zika virus disease epidemic: Second update Stockholm: ECDC http://ecdc.europa.eu/en/publications/_layouts/forms/Publication_DispForm.aspx?List=4f55ad51-4aed-4d32-b960-af70113dbb90&ID=1434
Fan Z, Li W, Lee SR, Meng Q, Shi B, Bunch TD, White KL, Kong IK, Wang Z (2014) Efficient gene targeting in golden Syrian hamsters by the CRISPR/Cas9 system. PLoS One 9(10):e109755. doi:10.1371/journal.pone.0109755
Fauci AS (2016) Testimony before the house democratic steering and policy committee D. O. H. A. H. Services and N. I. O. Health. National Institute of Allergy and Infectious Diseases
Faye O, Freire CC, Iamarino A, Faye O, de Oliveira JV, Diallo M, Zanotto PM, Sall AA (2014) Molecular evolution of Zika virus during its emergence in the 20(th) century. PLoS Negl Trop Dis 8(1):e2636. doi:10.1371/journal.pntd.0002636
Finlay BL, Darlington RB (1995) Linked regularities in the development and evolution of mammalian brains. Science 268(5217):1578–1584
Fontes CA, Dos Santos AA, Marchiori E (2016) Magnetic resonance imaging findings in Guillain-Barré syndrome caused by Zika virus infection. Neuroradiology 58(8):837–838. doi:10.1007/s00234-016-1687-9
Fox M (2015) This virus you never heard of may be causing birth defects in Brazil. NBC News http://www.nbcnews.com/health/health-news/virus-you-never-heard-may-be-causing-birth-defects-brazil-n474346. Accessed 6 Dec 2015
Fox M (2016) FDA says test of genetically modified mosquitoes is safe. NBC News http://www.nbcnews.com/storyline/zika-virus-outbreak/fda-says-test-genetically-modified-mosquitoes-safe-n536861. Accessed 11 Mar 2016
Foy BD, Kobylinski KC, Chilson Foy JL, Blitvich BJ, Travassos da Rosa A, Haddow AD, Lanciotti RS, Tesh RB (2011) Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis 17(5):880–882. doi:10.3201/eid1705.101939
Gao D, Lou Y, He D, Porco TC, Kuang Y, Chowell G, Ruan S (2016) Prevention and control of Zika as a mosquito-borne and sexually transmitted disease: a mathematical modeling analysis. Sci Report 6. doi:10.1038/srep28070
Gao M, Zhang B, Liu J, Guo X, Li H, Wang T, Zhang Z, Liao J, Cong N, Wang Y, Yu L (2014) Generation of transgenic golden Syrian hamsters. Cell Res 24(3):380. doi:10.1038/cr.2014.2
Garcez PP, Loiola EC, Madeiro da Costa R, Higa LM, Trindade P, Delvecchio R, Nascimento JM, Brindeiro R, Tanuri A, Rehen SK (2016) Zika virus impairs growth in human neurospheres and brain organoids. Science 352(6287):816–818. doi:10.1126/science.aaf6116
Gatherer D, Kohl A (2016) Zika virus: a previously slow pandemic spreads rapidly through the Americas. J Gen Virol 97(2):269–273. doi:10.1099/jgv.0.000381
Goodfellow FT, Tesla B, Simchick G, Zhao Q, Hodge T, Brindley MA, Stice SL (2016) Zika virus induced mortality and microcephaly in chicken embryos. Stem Cells Dev 25(22):1691–1697
Gourinat AC, O'Connor O, Calvez E, Goarant C, Dupont-Rouzeyrol M (2015) Detection of Zika virus in urine. Emerg Infect Dis 21(1):84–86. doi:10.3201/eid2101.140894
Halstead SB, Russell PK (2016) Protective and immunological behavior of chimeric yellow fever dengue vaccine. Vaccine 34(14):1643–1647. doi:10.1016/j.vaccine.2016.02.004
Hamel R, Dejarnac O, Wichit S, Ekchariyawat P, Neyret A, Luplertlop N, Perera-Lecoin M, Surasombatpattana P, Talignani L, Thomas F, Cao-Lormeau VM, Choumet V, Briant L, Desprès P, Amara A, Yssel H, Missé D (2015) Biology of Zika virus infection in human skin cells. J Virol 89(17):8880–8896. doi:10.1128/JVI.00354-15
Hancock WT, Marfel M, Bel M (2014) Zika virus, French Polynesia, South Pacific, 2013. Emerg Infect Dis 20(11):1960. doi:10.3201/eid2011.141380
Hayden EC (2016) The race is on to develop Zika vaccine. Nature http://www.nature.com/news/the-race-is-on-to-develop-zika-vaccine-1.19634 via the Internet. Accessed 28 Mar 2016
Hayes EB (2009 Sep) (2009) Zika Virus Outside Africa. Emerg Infect Dis 15(9):1347–1350. doi:10.3201/eid1509.090442
Hulo C, de Castro E, Masson P, Bougueleret L, Bairoch A, Xenarios I, Le Mercier P (2011) ViralZone: a knowledge resource to understand virus diversity. Nucleic Acids Res 39(Database issue):D576–D582. doi:10.1093/nar/gkq901
Ishikawa T, Yamanaka A, Konishi E (2014) A review of successful flavivirus vaccines and the problems with those flaviviruses for which vaccines are not yet available. Vaccine 32(12):1326–1337. doi:10.1016/j.vaccine.2014.01.040
Kaushik A, Tiwari S, Jayant RD, Vashist A, Moshaie RN, El-Hage N, Nair M (2016) Electrochemical biosensors for early stage Zika diagnostics. Trends Biotechnol doi.org/10.1016/j.tibtech.2016.10.001
Kenney J, Brault A (2014) The role of environmental, virological and vector interactions in dictating biological transmission of arthropod-borne viruses by mosquitoes. Adv Virus Res 89:39–83. doi:10.1016/B978-0-12-800172-1.00002-1
Kilpatrick AM, Randolph SE (2012) Drivers, dynamics, and control of emerging vector-borne zoonotic diseases. Lancet 380(9857):1946–1955. doi:10.1016/S0140-6736(12)61151-9
Kimura T, Sasaki M, Okumura M, Kim E, Sawa H (2010) Flavivirus encephalitis pathological aspects of mouse and other animal models. Vet Pathol Online 47(5):806–818. doi:10.1177/0300985810372507
Koide F, Goebel S, Snyder B, Walters KB, Gast A, Hagelin K, Kalkeri R, Rayner J (2016) Development of a Zika virus infection model in Cynomolgus macaques. Front Microbiol 7:2028 doi:10.3389/fmicb.2016.02028
Kraemer MU, Sinka ME, Duda KA, Mylne AQ, Shearer FM, Barker CM, Moore CG, Carvalho RG, Coelho GE, Van Bortel W, Hendrickx G, Schaffner F, Elyazar IR, Teng HJ, Brady OJ, Messina JP, Pigott DM, Scott TW, Smith DL, Wint GR, Golding N, Hay S (2015) The global distribution of the arbovirus vectors Aedes aegypti and ae. albopictus. Elife 30(4):e08347. doi:10.7554/eLife.08347
Kuno G (2001) Transmission of arboviruses without involvement of arthropod vectors. Acta Virol 45(3):139–150
Kuno G, Chang GJ (2007) Full-length sequencing and genomic characterization of Bagaza, Kedougou, and Zika viruses. Arch Virol 152(4):687–696
Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, Stanfield SM, Duffy MR (2008) Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 14(8):1232–1239. doi:10.3201/eid1408.080287
Lazear HM, Govero J, Smith AM, Platt DJ, Fernandez E, Miner JJ, Diamond MS (2016) A mouse model of Zika virus pathogenesis. Cell Host Microbe 19(5):720–730. doi:10.1016/j.chom.2016.03.010
Lee VH, Moore DL (1972) Vectors of the 1969 yellow fever epidemic on the Jos plateau, Nigeria. Bull World Health Organ 46(5):669–673
Li H, Saucedo-Cuevas L, Regla-Nava JA, Chai G, Sheets N, Tang W, Terskikh AV, Shresta S, Gleeson JG (2016b) Zika virus infects neural progenitors in the adult mouse brain and alters proliferation. Cell Stem Cell 19(5):593–598. doi:10.1016/j.stem.2016.08.005
Li C, Xu D, Ye Q, Hong S, Jiang Y, Liu X, Zhang N, Shi L, Qin CF, Xu Z (2016a) Zika virus disrupts neural progenitor development and leads to microcephaly in mice. Cell Stem Cell 19(5):672. doi:10.1016/j.stem.2016.10.017
Liang Q, Luo Z, Zeng J, Chen W, Foo SS, Lee SA, Ge J, Wang S, Goldman SA, Zlokovic BV, Zhao Z, Jung JU (2016) Zika virus NS4A and NS4B proteins deregulate Akt-mTOR signaling in human fetal neural stem cells to inhibit neurogenesis and induce autophagy. Cell Stem Cell 19(5):663–671. doi:10.1016/j.stem.2016.07.019
Manangeeswaran M, Ireland DD, Verthelyi D (2016) Zika (PRVABC59) infection is associated with T cell infiltration and neurodegeneration in CNS of immunocompetent neonatal C57Bl/6 mice. PLoS Pathog 12(11):e1006004. doi:10.1371/journal.ppat.1006004
Marano G, Pupella S, Vaglio S, Liumbruno GM, Grazzini G (2016) Zika virus and the never-ending story of emerging pathogens and transfusion medicine. Blood Transfus 14(2):95–100. doi:10.2450/2015.0066-15
Marchette NJ, Garcia R, Rudnick A (1969) Isolation of Zika virus from Aedes aegypti mosquitoes in Malaysia. AmJTrop Med Hyg 18(3):411–415
Maron DF (2016) Zika vaccine could solve one problem while stoking another. Scientific American. https://www.scientificamerican.com/article/zika-vaccine-could-solve-one-problem-while-stoking-another/. Accessed 1 Apr 2016
Marrs C, Olson G, Saade G, Hankins G, Wen T, Patel J, Weaver S (2016) Zika virus and pregnancy: a review of the literature and clinical considerations. Am J Perinatol 33(7):625–639. doi:10.1055/s-0036-1580089
Marthiens V, Rujano MA, Pennetier C, Tessier S, Paul-Gilloteaux P, Basto R (2013) Centrosome amplification causes microcephaly. Nat Cell Biol 15(7):731–740. doi:10.1038/ncb2746
Mécharles S, Herrmann C, Poullain P, Tran TH, Deschamps N, Mathon G, Landais A, Breurec S, Lannuzel A (2016) Acute myelitis due to Zika virus infection. Lancet 387(10026):1481. doi:10.1016/S0140-6736(16)00644-9
Mendola P, Selevan SG, Gutter S, Rice D (2002) Environmental factors associated with a spectrum of neurodevelopmental deficits. Ment Retard Dev Disabil Res Rev 8(3):188–197
Miner JJ, Cao B, Govero J, Smith AM, Fernandez E, Cabrera OH, Garber C, Noll M, Klein RS, Noguchi KK, Mysorekar IU, Diamond MS (2016) Zika virus infection during pregnancy in mice causes placental damage and fetal demise. Cell 165(5):1081–1091. doi:10.1016/j.cell.2016.05.008
Mlakar J, Korva M, Tul N, Popović M, Poljšak-Prijatelj M, Mraz J, Kolenc M, Resman Rus K, Vesnaver Vipotnik T, Fabjan Vodušek V, Vizjak A, Pižem J, Petrovec M, Avšič Županc T (2016) Zika virus associated with microcephaly. N Engl J Med 374(10):951–958. doi:10.1056/NEJMoa1600651
Mochida GH (2009) Genetics and biology of microcephaly and lissencephaly. Semin Pediatr Neurol 16(3):120–126. doi:10.1016/j.spen.2009.07.001
Mochida GH, Walsh CA (2001) Molecular genetics of human microcephaly. Curr Opin Neurol 14(2):151–156
Morrison TE, Diamond MS (2017) Animal models of Zika virus infection, pathogenesis, and immunity. J Virol JVI-00009. doi:10.1128/JVI.00009-17
Mukhopadhyay S, Kuhn RJ, Rossmann MG (2005) A structural perspective of the flavivirus life cycle. Nat Rev Microbiol 3(1):13–22
Musso D, Roche C, Robin E, Nhan T, Teissier A, Cao-Lormeau VM (2015) Potential sexual transmission of Zika virus. Emerg Infect Dis 21(2):359–361. doi:10.3201/eid2102.141363
Nabel GJ (2013) Designing tomorrow's vaccines. N Engl J Med 368(6):551–560. doi:10.1056/NEJMra1204186
Nah JJ, Yang DK, Kim HH, Song JY (2015) The present and future of veterinary vaccines for Japanese encephalitis in Korea. Clin Exp Vaccine Res 4(2):130–136. doi:10.7774/cevr.2015.4.2.130
Ngono AE, Vizcarra EA, Tang WW, Sheets N, Joo Y, Kim K, Gorman MJ, Diamond MS, Shresta S (2017) Mapping and role of the CD8+ T cell response during primary Zika virus infection in mice. Cell Host Microbe 21(1):35–46. doi:10.1016/j.chom.2016.12.010
Nigg EA, Stearns T (2011) The centrosome cycle: centriole biogenesis, duplication and inherent asymmetries. Nat Cell Biol 13(10):1154–1160. doi:10.1038/ncb2345
Oliveira Melo AS, Malinger G, Ximenes R, Szejnfeld PO, Alves Sampaio S, Bispo de Filippis AM (2016) Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound Obstet Gynecol 47(1):6–7. doi:10.1002/uog.15831
Onorati M, Li Z, Liu F, Sousa AM, Nakagawa N, Li M, Dell'Anno MT, Gulden FO, Pochareddy S, Tebbenkamp AT, Han W, Pletikos M, Gao T, Zhu Y, Bichsel C, Varela L, Szigeti-Buck K, Lisgo S, Zhang Y, Testen A, Gao XB, Mlakar J, Popovic M, Flamand M, Strittmatter SM, Kaczmarek LK, Anton ES, Horvath TL, Lindenbach BD, Sestan N (2016) Zika virus disrupts Phospho-TBK1 localization and mitosis in human neuroepithelial stem cells and radial glia. Cell Rep 16(10):2576–2592. doi:10.1016/j.celrep.2016.08.038
Oster AM (2016) Interim guidelines for prevention of sexual transmission of Zika virus—United States. Morb Mortal Wkly Rep 65:120
Osuna CE, Lim SY, Deleage C, Griffin BD, Stein D, Schroeder LT, Omage R, Best K, Luo M, Hraber PT, Andersen-Elyard H (2016) Zika viral dynamics and shedding in rhesus and cynomolgus macaques. Nat Med. doi:10.1038/nm.4206
Pan American Health Organization / World Health Organization (PAHO/WHO) (2015) Neurological syndrome, congenital malformations, and zika virus infection. Implications for public health in the Americas- Epidemiological Alert. http://www.paho.org/hq/index.php?option=com_topics&view=readall&cid=7880&Itemid=41484&lang=en. Accessed 01 Dec 2015
Pardee K, Green AA, Takahashi MK, Braff D, Lambert G, Lee JW, Ferrante T, Ma D, Donghia N, Fan M, Daringer NM, Bosch I, Dudley DM, O'Connor DH, Gehrke L, Collins JJ (2016) Rapid, low-cost detection of Zika virus using programmable biomolecular components. Cell 165(5):1255–1266. doi:10.1016/j.cell.2016.04.059
Sanofi Pasteur (2016). Sanofi Pasteur to leverage its strong vaccine legacy in hunt for Zika vaccine. Press Release http://www.sanofipasteur.com/en/articles/Sanofi-Pasteur-to-leverage-its-strong-vaccine-legacy-in-hunt-for-Zika-vaccine.aspx. Accessed 2 Feb 2016
Petersen LR, Jamieson DJ, Powers AM, Honein MA (2016) Zika Virus. N Engl J Med 21;374(16):1552-63. doi:10.1056/NEJMra1602113.
Pierson TC, Kielian M (2013) Flaviviruses: braking the entering. Curr Opin Virol 3(1):3–12. doi:10.1016/j.coviro.2012.12.001
Plotkin SA (2016) Zika as still another argument for a new path to vaccine development. Clin Microbiol Infect 22(4):294–295. doi:10.1016/j.cmi.2016.03.001
Plotkin SA, Mahmoud AA, Farrar J (2015) Establishing a global vaccine-development fund. N Engl J Med 373(4):297–300. doi:10.1056/NEJMp1506820
Powell JR, Tabachnick WJ (2013) History of domestication and spread of Aedes aegypti - a review. Mem Inst Oswaldo Cruz 1:11–17. doi:10.1590/0074-0276130395
Pronker ES, Weenen TC, Commandeur H, Claassen EH, Osterhaus AD (2013) Risk in vaccine research and development quantified. PLoS One 8(3):e57755. doi:10.1371/journal.pone.0057755
Pylro VS, Oliveira FS, Morais DK, Cuadros-Orellana S, Pais FS, Medeiros JD, Geraldo JA, Gilbert J, Volpini AC, Fernandes GR (2016) ZIKV - CDB: a collaborative database to guide research linking SncRNAs and ZIKA virus disease symptoms. PLoS Negl Trop Dis 10(6):e0004817. doi:10.1371/journal.pntd.0004817.
Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, Hammack C, Yao B, Hamersky GR, Jacob F, Zhong C, Yoon KJ, Jeang W, Lin L, Li Y, Thakor J, Berg DA, Zhang C, Kang E, Chickering M, Nauen D, Ho CY, Wen Z, Christian KM, Shi PY, Maher BJ, Wu H, Jin P, Tang H, Song H, Ming GL (2016) Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 165(5):1238-54. doi: 10.1016/j.cell.2016.04.032.
Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR (2016) Zika virus and birth defects—reviewing the evidence for causality. N Engl J Med 374(20):1981–1987. doi:10.1056/NEJMsr1604338
Roehrig JT, Hombach J, Barrett AD (2008) Guidelines for plaque-reduction neutralization testing of human antibodies to dengue viruses. Viral Immunol 21(2):123–132. doi:10.1089/vim.2008.0007
Rossi SL, Tesh RB, Azar SR, Muruato AE, Hanley KA, Auguste AJ, Langsjoen RM, Paessler S, Vasilakis N, Weaver SC (2016) Characterization of a novel murine model to study Zika virus. Am J Trop Med Hyg 94(6):1362–1369. doi:10.4269/ajtmh.16-0111
Sapparapu G, Fernandez E, Kose N, Cao B, Fox JM, Bombardi RG, Zhao H, Nelson CA, Bryan AL, Barnes T, Davidson E (2016) Neutralizing human antibodies prevent Zika virus replication and fetal disease in mice. Nature 540(7633):443–447. doi:10.1038/nature20564
Sarno M, Sacramento GA, Khouri R, do Rosário MS, Costa F, Archanjo G, Santos LA, Nery N Jr, Vasilakis N, Ko AI, de Almeida AR (2016) Zika virus infection and stillbirths: a case of hydrops fetalis, hydranencephaly and fetal demise. PLoS Negl Trop Dis 10(2):e0004517. doi:10.1371/journal.pntd.0004517
Schuler-Faccini L, Ribeiro EM, Feitosa IM, Horovitz DD, Cavalcanti DP, Pessoa A, Doriqui MJ, Neri JI, Neto JM, Wanderley HY, Cernach M, El-Husny AS, Pone MV, Serao CL, Sanseverino MT, Brazilian Medical Genetics Society–Zika Embryopathy Task Force (2015) Possible association between Zika virus and microcephaly - Brazil. MMWR Morb Mortal Wkly Rep 65(3):59–62. doi:10.15585/mmwr.mm6503e2
Shah A, Kumar A (2016) Zika virus infection and development of a murine model. Neurotox Res 30(2):131–134. doi:10.1007/s12640-016-9635-3
Shao Q, Herrlinger S, Yang SL, Lai F, Moore JM, Brindley MA, Chen JF (2016) Zika virus infection disrupts neurovascular development and results in postnatal microcephaly with brain damage. Development 143(22):4127–4136
Shuaib W, Stanazai H, Abazid AG, Mattar AA (2016) Re-emergence of Zika virus: a review on pathogenesis, clinical manifestations, diagnosis, treatment, and prevention. Am J Med 129(8):879.e7–879.e12. doi:10.1016/j.amjmed.2016.02.027
Stettler K, Beltramello M, Espinosa DA, Graham V, Cassotta A, Bianchi S, Vanzetta F, Minola A, Jaconi S, Mele F, Foglierini M (2016) Specificity, cross-reactivity and function of antibodies elicited by Zika virus infection. Science. doi:10.1126/science.aaf8505
Sun LH (2016) Zika: more than 2,500 babies born with microcephaly in Brazil, WHO predicts. The Wahington post. https://www.washingtonpost.com/news/to-your-health/wp/2016/03/22/zika-in-brazil-more-than-2500-births-with-microcephaly-whopredicts/?utm_term=.931909a9d7b4. Accessed 22 Mar 2016
Suthar MS, Diamond MS, Gale M Jr (2013) West Nile virus infection and immunity. Nat Rev Microbiol 11(2):115–128. doi:10.1038/nrmicro2950
Tang H, Hammack C, Ogden SC, Wen Z, Qian X, Li Y, Yao B, Shin J, Zhang F, Lee EM, Christian KM, Didier RA, Jin P, Song H, Ming GL (2016) Zika virus infects human cortical neural progenitors and attenuates their growth. Cell Stem Cell 18(5):587–590. doi:10.1016/j.stem.2016.02.016
Tang-Wing C, Dadelahi A, Balaraman V, Kantor A, Franz A, Adamovicz J (n.d.) Aedes aegypti Mosquito transmission and the development of STAT2 KO hamster animal model for Zika Virus. http://vrsp.missouri.edu/wp-content/uploads/2017/01/Tang-Wing.pdf
Tesh RB, Siirin M, Guzman H, da Rosa AP, Wu X, Duan T, Lei H, Nunes MR, Xiao SY (2005) Persistent West Nile virus infection in the golden hamster: studies on its mechanism and possible implications for other flavivirus infections. J Infect Dis 192(2):287–295. doi:10.1086/431153
Tetro JA (2016) Zika and microcephaly: causation, correlation, or coincidence? Microbes Infect 18(3):167–168. doi:10.1016/j.micinf.2015.12.010
Thornton GK, Woods CG (2009) Primary microcephaly: do all roads lead to Rome? Trends Genet 25(11):501–510. doi:10.1016/j.tig.2009.09.011
Tonry JH, Xiao SY, Siirin M, Chen H, DA ROSA AP, Tesh RB (2005) Persistent shedding of West Nile virus in urine of experimentally infected hamsters. AmJTrop Med Hyg 72(3):320–324
Unni SK, Růžek D, Chhatbar C, Mishra R, Johri MK, Singh SK (2011) Japanese encephalitis virus: from genome to infectome. Microbes Infect 13(4):312–321
Ventura CV, Maia M, Bravo-Filho V, Góis AL, Belfort R Jr (2016) Zika virus in Brazil and macular atrophy in a child with microcephaly. Lancet 387(10015):228. doi:10.1016/S0140-6736(16)00006-4
Venturi G, Zammarchi L, Fortuna C, Remoli ME, Benedetti E, Fiorentini C, Trotta M, Rizzo C, Mantella A, Rezza G, Bartoloni A (2016) An autochthonous case of Zika due to possible sexual transmission, Florence, Italy, 2014. Euro Surveill 21(8):30148. doi:10.2807/1560-7917
Wang H, Siddharthan V, Hall JO, Morrey JD (2009) West Nile virus preferentially transports along motor neuron axons after sciatic nerve injection of hamsters. J Neuro-Oncol 15:293–299. doi:10.1080/13550280902973978
Weaver SC, Costa F, Garcia-Blanco MA, Ko AI, Ribeiro GS, Saade G, Shi PY, Vasilakis N (2016) Zika virus: history, emergence, biology, and prospects for control. Antivir Res 130:69–80. doi:10.1016/j.antiviral.2016.03.010
Weinbren MP, Williams MC (1958) Zika virus: further isolations in the zika area, and some studies on the strains isolated. Trans R Soc Trop Med Hyg 52(3):263–268
Wong PS, Li MZ, Chong CS, Ng LC, Tan CH (2013) Aedes (Stegomyia) albopictus (Skuse): a potential vector of Zika virus in Singapore. PLoS Negl Trop Dis 7(8):e2348. doi:10.1371/journal.pntd.0002348
World Health Organization (2016) Surveillance for Zika virus infection, microcephaly and Guillain-Barré syndrome. Interim guidance WHO/ZIKV/SUR/16.2 Rev.1, 9. http://www.who.int/csr/resources/publications/zika/surveillance/en/. Accessed 7 Apr 2016
Wu KY, Zuo GL, Li XF, Ye Q, Deng YQ, Huang XY, Cao WC, Qin CF, Luo ZG (2016) Vertical transmission of Zika virus targeting the radial glial cells affects cortex development of offspring mice. Cell Res 26(6):645–654. doi:10.1038/cr.2016.58
Xiao SY, Guzman H, Zhang H, Travassos da Rosa AP, Tesh RB (2001) West Nile virus infection in the golden hamster (Mesocricetus auratus): a model for West Nile encephalitis. Emerg Infect Dis 7:714–721. doi:10.3201/eid0704.010420
Zmurko J, Marques RE, Schols D, Verbeken E, Kaptein SJ, Neyts J (2016) The viral polymerase inhibitor 7-Deaza-2′-C-Methyladenosine is a potent inhibitor of in vitro Zika virus replication and delays disease progression in a robust mouse infection model. PLoS Negl Trop Dis 10(5):e0004695. doi:10.1371/journal.pntd.0004695
Acknowledgements
This review was written as part of the curriculum for the course GMS 6939, offered to the Ph.D. graduate students in the Biomedical Science Program at Florida International University. We gratefully acknowledge the support of the Florida Department of Health: Zika Research Grant Initiative # 7ZK09 and the National Institutes of Health (NIH) grants R01 DA036154 to NEH and JL; R01 DA036154-S1 to JL; R01 DA034547 to AK and ST; R01 ES023779 to SP and JJL; R01 GM055425 to SP; and R15 AI111210 to MP and KM; the National Institute of General Medical Sciences (NIGMS) Research Initiative for Scientific Enhancement (RISE) Program R25 GM061347 to MRS; the National Science Foundation (NSF) 1408063 to AR; Florida International University Graduate School Presidential Fellowship to CO and TP; the McKnight Fellowship to LG; and the Herbert Wertheim College of Medicine Teaching Assistance to SD, RD, IP and AC. SP, AK, and NEH critically revised the work and figures for important intellectual content. In addition, we thank Luis Garbinski, Allen Caobi and Mairyn Piloto, in the Biomedical Sciences Program at Florida International University, for assisting with the animal models depicted in Table 2.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Pawitwar, S.S., Dhar, S., Tiwari, S. et al. Overview on the Current Status of Zika Virus Pathogenesis and Animal Related Research. J Neuroimmune Pharmacol 12, 371–388 (2017). https://doi.org/10.1007/s11481-017-9743-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-017-9743-8