Abstract
The evaluation and management of methamphetamine-associated psychosis (MAP) is an area of study with a paucity of large-scale, longitudinal data. Methamphetamine use has soared in popularity worldwide in the past decade, leading to a surge in individuals experiencing its neurotoxic effects. Current evidence suggests that methamphetamine causes neurodegeneration and psychosis through VMAT2 inhibition which raises dopamine and GABA levels in the brain’s dopaminergic pathways, leading to oxidative stress and inflammation. Differentiating MAP from primary psychotic disorders is challenging; high rates of persistent psychosis leading to a diagnosis of primary psychotic disorder and an absence of an etiologic differentiation amongst the DSM-5 diagnostic criteria further complicate the diagnostic process. Once a diagnosis of methamphetamine-associated psychosis is made, benzodiazepines have been shown to provide temporary relief; in addition, depending on the severity and impact of psychotic symptoms, antipsychotics may be indicated both short and long terms for ongoing symptom management. Robust data for these treatments is limited and primarily draws on animal studies or case reports. Further research is needed to codify MAP treatment standards of care.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Stimulant use, including methamphetamine, 3,4-methyl enedioxy-methamphetamine (MDMA), and cocaine, constitutes a growing prevalence worldwide. These highly potent substances have numerous negative biopsychosocial impacts including homelessness, intravenous drug use, cardiovascular morbidity, drug-seeking sex work, and long-term psychiatric symptoms (Barr et al., 2006). Methamphetamine use, after rising steadily in the 1990s and declining in the early 2000s, has risen to all-time highs (Substance Abuse and Mental Health Services Administration (SAMHSA), 2023). Use patterns, availability, and health consequence are a function of the type and abundance of methamphetamine available, largely produced via a phenyl-2-proponone (P2P) method. Given the relative ease with which the precursor P2P can be obtained, methamphetamine is produced on a widespread industrial scale, which results in ubiquitous availability at historically low prices. Moreover, P2P methamphetamine, compared to alternatives prepared from ephedrine or pseudoephedrine, seems to pose unique health risks, including worse neurotoxicity and psychosis (Courtney & Ray, 2014). Most recent epidemiological data indicate that in 2021, over 2.5 million people in the USA reported methamphetamine use that year, with over 16 million people reporting a lifetime history (SAMSHA 2023). Despite these statistics, only about 17.8% of individuals in drug treatment programs in the USA are enrolled for the treatment of stimulant use (Ronsley et al., 2020).
Current modes of treatment for stimulant use disorder are varied, encompassing social and pharmacological methods, with the most robust evidence-based treatment endorsing contingency management for reduction in psychostimulant use (Ronsley et al., 2020). Pharmacotherapy options have been explored for treating methamphetamine use disorder including opioid antagonists (Jayaram-Lindström et al., 2008), anticonvulsants (Elkashef et al., 2012), psychostimulants (Konstenius et al., 2014), and antidepressants (Naji et al., 2022), but the results have provided insufficient evidence of significant benefit (Chan et al., 2019). Despite the availability of research studying methamphetamine use disorder, research specifically related to methamphetamine-associated psychosis (MAP) and evidence-based treatments are lacking. Studies conducted in Japan show that psychosis is a common effect of methamphetamine use with up to 21% of users experiencing psychosis that persists more than 6 months after discontinuing use, and 49% experienced psychotic relapse over the course of 15–20 months (Barr et al., 2006). Studies have reported that approximately 15–23% of recreational methamphetamine users experience MAP (McKetin et al., 2006, 2010), and up to 60% of people with methamphetamine use disorder (previously known as dependence) experience psychosis (Smith et al., 2009; Sulaiman et al., 2014).
Pharmacotherapy is often employed in MAP treatment (Chiang et al., 2019; Fluyau et al., 2019), but evidence regarding specific medications, dosage, and treatment duration is limited. Therefore, the present review aims to provide updated, clinically focused information on the differentiation between methamphetamine-associated psychosis and primary psychotic disorders, along with the proposed neurobiological effects of MAP and evidence-based treatment.
Methods
The authors conducted electronic searches, using PubMed, Google Scholar, and PsycINFO. Search parameters included all English-language articles published up to April 2023. Search terms included “methamphetamine” (or “amphetamine”) in conjunction with terms such as “psychosis,” “substance induced,” “treatment,” “stimulant induced,” “persistent,” and “neurobiology.” Among search results with relevant titles, abstracts were reviewed. Full-text articles were retrieved for further investigation as determined by authors. All relevant randomized controlled trials, retrospective cohort studies, and review articles were included. References in retrieved articles were reviewed for additional relevant articles. Manuscripts that were not subject to peer-review as well as literature published in languages other than English were excluded.
Results
Neurotoxicity of Methamphetamine
Once metabolized, methamphetamine increases dopamine levels in the central nervous system through the inhibition of dopamine transporters (DAT) and the vesicular monoamine transporter (VMAT2) (Chiang et al., 2019). VMAT2 inhibition causes an increased concentration of dopamine in the three dopaminergic pathways: nigrostriatal, which is primarily involved in behaviors associated with reward or predicted stimuli; mesolimbic, which aids in processing rewards and translating reward-associated emotions into action; and mesocortical, which is thought to primarily affect cognitive function (Hsieh et al., 2014). Without the activity of the dopamine and VMAT2 transporters, dopamine is also released from the ventral tegmental area and travels to the nucleus accumbens and pre-frontal cortex of the mesolimbic dopaminergic pathway, where it increases to neurotoxic levels (Jan et al., 2012). Eventually, the dopaminergic receptor density and function adjust to chronically heightened dopamine signaling (Chiang et al., 2019; Hsieh et al., 2014). Activation of dopamine in the three pathways causes increased release of gamma-aminobutyric acid (GABA) from the substantia nigra pars reticulata of the brain, and subsequent disinhibition of glutamate release within the cortex (Chiang et al., 2019; Hsieh et al., 2014). An increase in glutamate reinforces this process, allowing the cycle to perpetuate for over 24 h after methamphetamine use (Hsieh et al., 2014). The dysregulation in dopaminergic and GABA signaling in the cortex is one of the mechanisms believed to trigger the disorganized thought process and hallucinations associated with MAP through the damage of cortical interneurons (Chiang et al., 2019; Hsieh et al., 2014).
In addition to the relationship between neurotransmitter dysregulation and psychotic symptoms, inflammation and oxidative stress in the brain are also theorized to exacerbate MAP. As an individual uses methamphetamine, the blood brain barrier is compromised by a methamphetamine-induced influx of pro-inflammatory cytokines and a decrease of pro-inflammatory cytokines, which are cytotoxic to the neurons (Licinio et al., 1993; Papageorgiou et al., 2019; Srisurapanont et al., 2021; X. Yang et al., 2020a, b). High levels of inflammatory cytokines have been correlated with more severe psychosis and cognitive dysfunction in patients with MAP (X. Yang et al., 2020a, b). The inflammatory cytokine, IL-2, can provoke dopamine release (Lapchak, 1992), and therefore may contribute to the dopamine excess that causes psychotic symptoms (Licinio et al., 1993), as well as triggering an overproduction of reactive oxygen species, thereby becoming neurotoxic and causing apoptosis of neurons involved in dopaminergic signaling (Chiang et al., 2019; Jan et al., 2012). This increased oxidative burden occurs via methamphetamine-induced morphologic changes in mitochondria and DNA strand breaks (Paulus & Stewart, 2020).
While methamphetamine leads to microscopic degeneration, it also can cause gross changes in the brain. Studies have indicated that those who have experienced MAP, compared to methamphetamine users who have not experienced psychosis, have less cortical thickness in areas of the brain where substance use dysregulation is theorized to occur (Srisurapanont et al., 2021).
Risk Factors
Individuals with MAP were 5 times more likely to have a family history of schizophrenia compared to methamphetamine users without psychosis (Seeman, 2005), suggesting a relationship between the hereditary factors for schizophrenia and MAP. Multiple genetic variations commonly found in individuals with schizophrenia have also been found significantly more frequently in patients with MAP compared to healthy controls (Kishimoto et al., 2008) and compared to methamphetamine users without psychosis (Breen et al., 2016). In addition to possible genetic predisposition for MAP, shared risk factors between substance abuse and mental illness, such as environmental exposures, neurological mechanisms, impulsivity, and social pressures, may play a role in development of MAP (Kuitunen-Paul et al., 2020). For example, childhood trauma may be a risk factor for psychosis among methamphetamine users; individuals that reported three or more adverse childhood events (ACEs) had 4.5 times higher odds of a lifetime psychosis compared to methamphetamine users without reported ACEs (Ding et al., 2014). It has been reported that regular methamphetamine users were 11 times more likely to experience psychosis than the general population (Lappin & Sara, 2019), and the risk of MAP is higher among methamphetamine users with earlier age of initiation of methamphetamine use, larger doses of methamphetamine, longer duration of drug use, or more frequent methamphetamine use (Cumming et al., 2020; Jones et al., 2020; M. Yang et al., 2020a, b). Additionally, Yang and colleagues found that there is a positive correlation between methamphetamine use duration and MAP duration (M. Yang et al., 2020a, b).
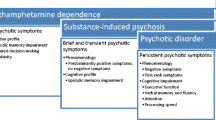
Definitions
Acute Methamphetamine-Associated Psychosis
Acute methamphetamine-associated psychosis can be diagnosed when the psychotic symptoms emerge during or within 1 month of usage of the drug, commonly showing signs of irritability, anxiety, psychosis, and mood disturbances with the prominent psychotic symptoms being auditory and tactile hallucinations, ideas of reference, and paranoid delusions (Zweben et al., 2004; M. Yang et al., 2020a, b). In a study conducted by Fasihpour et al. (2013), in an inpatient setting, the predominant symptoms were persecutory delusions (82%), auditory hallucinations (70.3%), reference delusions (57.7%), visual hallucination (44.1%), grandiosity delusions (39.6%), infidelity delusions (26.1%), bizarre delusions (7.2%), thought broadcasting (6.3%), passivity feelings (4.5%), thought withdrawals (3.6%), tactile hallucinations (1.8%), thought insertions (1.8%), olfactory hallucinations (0.9%), and nihilistic delusions (0.9%). (Fasihpour et al., 2013).
Chronic Methamphetamine-Associated Psychosis
Methamphetamine-associated psychosis was initially believed to follow a transient course, with symptoms fading with time since last use, but approximately 16–25% of methamphetamine users experience chronic MAP that lasts 1 month or more beyond achieving abstinence (Fiorentini et al., 2021; Iwanami et al., 1994; McKetin et al., 2016; Su et al., 2018; Voce et al., 2019a, b), and one study reported patients that experienced symptoms similar to schizophrenia after 8 to 12 years of abstinence (Teraoka, 1967). Chronic MAP manifests with chronic symptoms including ideas of reference, delusions, and hallucinations (Akiyama et al., 2011; M. Yang et al., 2020a, b). These findings suggest that methamphetamine-associated psychosis is not always limited to a transient or acute course and can instead persist chronically in a significant portion of patients.
Schizophrenia as a Comparison
Due to the overlap in symptoms, schizophrenia should be included in the differential of MAP. These similarities can be seen in the diagnostic criteria for schizophrenia, found in the DSM-5-TR, replicated in Table 1 (American Psychiatric Association, 2022). Looking at the pathophysiology of schizophrenia, it is clear why this is the case. Dopamine and glutamate are the leading neurotransmitters implicated in schizophrenia (Jauhar et al., 2022). Increased dopamine synthesis has been frequently replicated in studies looking at patients with schizophrenia, which correlates with the symptoms seen in patients with MAP. Another hypothesis is glutamate deficiency, illustrated in recreational phencyclidine (an anesthetic drug) use which blocks the glutamatergic postsynaptic receptor N-methyl-D-aspartate (NMDA) and is known to cause lengthy periods of intense psychosis (Jauhar et al., 2022).
Comparison Between Acute Methamphetamine Psychosis and Primary Psychotic Disorder
The diagnosis of acute methamphetamine-associated psychosis can usually be differentiated from a primary psychotic disorder based on DSM 5-TR criteria replicated in Table 2. Additionally, researchers have observed differences in the symptoms and severity of symptoms across both conditions. Close observation and comparison of symptoms across both conditions revealed the predominance of persecutory delusions, auditory and visual hallucinations, odd speech, and delusions of reference in methamphetamine-associated psychosis relative to primary psychotic disorder (Chen et al., 2003). Shelly et al. reported that those with acute amphetamine psychosis showed higher frequency of auditory hallucinations (48.5%) in comparison to schizophrenia (20.3%), whereas thought broadcasting was less prevalent in the MAP group (24%) compared to the schizophrenia group (Shelly et al., 2016). Several studies have also shown the predominance of thought disorder (characterized by the loosening of associations and disorganized speech) in schizophrenia and have established the presence of thought disorder as a salient and defining feature of schizophrenia, as these symptoms are rarely ever seen in methamphetamine-associated psychosis (Angrist et al., 1974; BELL, 1965; Yui et al., 2000). Studies have also reported differences in the severity of negative symptoms as a differentiating factor between schizophrenia and MAP, with some studies demonstrating fewer negative symptoms in patients with MAP compared to those with SSD (Hajebi et al., 2018; M. Yang et al., 2020a, b). Hajebi et al. compared the severity of the symptoms and found that those with non-affective psychosis presented with severe negative symptoms relative to those with acute methamphetamine psychosis and that those in the non- affective psychosis group continued to maintain increased severity of negative symptoms after discharge (Hajebi et al., 2018). However, the literature on this subject has been discordant; Srisurapanont et al. found no significant difference in symptom severity for positive or negative symptoms between the schizophrenia and MAP groups (Srisurapanont et al., 2011), highlighting a need for further research to compare these two disorders.
The trials that compared the differences in the cognitive symptoms between methamphetamine-associated psychosis and schizophrenia reported that both methamphetamine-associated psychosis and schizophrenia had similar cognitive symptoms profile (Chen et al., 2015; Jacobs et al., 2008), although Ezzatpanah et al. have also reported that visual attention deficits were more pronounced in schizophrenia compared to acute methamphetamine psychosis (Ezzatpanah et al., 2014). These findings led to the conclusion that certain brain areas (e.g., parietal cortex) were affected more in schizophrenia compared to acute methamphetamine psychosis (Wearne & Cornish, 2018).
Comparison Between Chronic Methamphetamine-Associated Psychosis and Primary Psychotic Disorder
The diagnosis of chronic methamphetamine-associated psychosis is complicated by various factors, such as the previously stated overlap in symptoms with schizophrenia spectrum disorder, relapse in methamphetamine use, and possible cessation of psychiatric medication (Hajebi et al., 2018). Studies conducted on patients with chronic psychosis have shown that the symptom profile of chronic methamphetamine psychosis overlaps with that of schizophrenia (Medhus et al., 2013; Srisurapanont et al., 2011). However, a 2016 study by Wang et al. found subtle differences in the symptom presentation which can be used to distinguish chronic methamphetamine psychosis from schizophrenia (L. J. Wang et al., 2016). This study compared 53 patients with chronic methamphetamine psychosis and 53 patients with schizophrenia and found that participants in the MAP group experienced higher proportions of visual hallucinations (30.2%) and somatic hallucinations (20.8%) than patients in the schizophrenia group (11.3% and 3.8%, respectively), while conceptual disorganization was more pronounced in schizophrenia group compared to chronic methamphetamine psychosis group (L. J. Wang et al., 2016). The patients in the schizophrenia group had a higher educational attainment than in the chronic methamphetamine psychosis group, suggesting that the differences in conceptual disorganization were not attributed to education level. It has also been found that schizophrenia was associated with greater frequency and severity of negative symptoms compared to chronic methamphetamine psychosis (M. Yang et al., 2020a, b). In addition to differences in symptom presentation between chronic MAP and SSD, one study also reported that treatment response to antipsychotic medication differs between patients with schizophrenia and chronic MAP, and thus could be one way to distinguish between these two diseases (Sekiguchi et al., 2021). In this study, patients with chronic MAP required fewer antipsychotic medications at lower chlorpromazine equivalent doses to manage their symptoms, as well as a shorter hospital stay, when compared to the SSD group (Sekiguchi et al., 2021). Studies differentiating chronic methamphetamine psychosis from acute methamphetamine psychosis reported that non-auditory hallucination occurred with greater frequency in chronic methamphetamine psychosis compared to acute methamphetamine psychosis (Iwanami et al., 1994). It has also been reported that the negative symptoms occurred with greater severity in patients with schizophrenia compared to those with acute and chronic methamphetamine psychosis, but the negative symptoms demonstrated by the chronic methamphetamine psychosis group were significantly greater than those in the acute methamphetamine group (Chen et al., 2003). Additionally, Voce and colleagues found that negative symptoms associated with MAP were more frequently found in patients who used methamphetamine in combination with other illicit substances compared to those without polysubstance use (Voce et al., 2019a, b). Methamphetamine can also cause toxic injury to the brain, and while trials have shown that MAP and schizophrenia have similar symptom profiles, some biological changes seen in methamphetamine psychosis may vary from those seen in schizophrenia (Yamamuro et al., 2015). Yamamuro et al. used functional near-infrared spectroscopy (NIRS) to monitor the brain blood oxygenation levels while performing verbal fluency tasks (VFT) in patients with methamphetamine psychosis and schizophrenia and found that oxyhemoglobin concentration changes in the prefrontal cortex (channels 8, 9, and 12) during VFT were significantly larger in patients with methamphetamine psychosis than they were in patients with schizophrenia (Yamamuro et al., 2015). However, despite these findings, many studies have found biological similarities between schizophrenia and MAP regarding genes (Breen et al., 2016; Kishimoto et al., 2008), brain structure volumes (Farnia et al., 2020), and cytokine aberrations (Dahan et al., 2018; Goldsmith et al., 2016; X. Yang et al., 2020a, b). When studied in a South-African population, however, no such genetic relationship was found; Asadi et al. found that polygenic risk scores for schizophrenia were not correlated with MAP nor any brain measures (Asadi et al., 2021), suggesting that further genetic studies in diverse populations are warranted. These findings suggest that the pathophysiology of schizophrenia and methamphetamine-associated psychosis share similar characteristics, but additional research may be useful to further explore pathophysiologic differences between the two diseases.
Treatment of Acute Map
Methamphetamine-associated psychotic symptoms can pose challenges to treatment providers, particularly if symptoms are acute and complex in nature or if agitation is involved. Furthermore, because the clinical presentation can overlap significantly with a primary psychotic disorder, securing an accurate diagnosis can prove challenging. However, obtaining the correct diagnosis can have treatment implications and can be important for discharge planning. For example, a patient with a primary psychotic disorder may benefit from case management services, while a patient with MAP may benefit from substance use disorder treatment to minimize future use of methamphetamines.
A careful history of the patient should also include the quantity, duration, and method of methamphetamine use as these factors are associated with MAP (Arunogiri et al., 2018; R et al., 2013). Studies suggest that binge use of methamphetamines is associated with psychotic symptoms (Glasner-Edwards & Mooney 2014; Lineberry & Bostwick, 2006). In addition, the onset of psychotic symptoms is associated with the duration and timing of methamphetamine use (Bramness et al., 2012).
Obtaining a history of other psychiatric symptoms also remains important. Irritability, anxiety, and mood changes can also occur with methamphetamine use (Zweben et al., 2004). Providers should observe for negative symptoms during a mental status exam, including signs of apathy (through blunted affect), avolition (lack of goal directed activity), alogia (decreased verbal spontaneity), and anhedonia (loss of pleasure). If available, laboratory workup can be considered including urine drug screen to assess for substance use, complete blood count, and metabolic panel to assess for infection, anemia, hypothyroidism or folate deficiency, and liver function tests to look for potential alcohol or IV drug use (Hepatitis C). Collateral history should be gathered to corroborate findings and establish a longitudinal view of the patient’s symptoms to better decide on appropriate diagnosis, treatment, and prognosis.
As there are few studies regarding treatment of MAP, treatment recommendations for clinicians are generally based upon case reports, animal studies, and the transference of knowledge in the treatment for acutely agitated patients. Benzodiazepines are effective in reducing the acute symptoms of MAP (Grigg et al., 2018; Richards et al., 2015) but are associated with sedation, tolerance, and respiratory depression, and may not be appropriate for long-term use (Johnson & Streltzer, 2013). In a randomized clinical trial in China, 120 patients with acute MAP symptoms were treated with either paliperidone extended release (ER) or risperidone; patients from both groups experienced statistically significant improvements in Positive and Negative Syndrome Scale (PANSS) total score following the treatment course, without statistically significant differences in efficacy between the two medications, and adverse effects were significantly reduced in the paliperidone ER group (G. Wang et al., 2020). Verachai and colleagues conducted a double-blind randomized controlled trial comparing the efficacy of quetiapine to haloperidol and found that quetiapine was as effective for the treatment of MAP as haloperidol, with comparable side effects (Verachai et al., 2014). These findings are similar to a study done by Leelahanaj in 2005, which compared olanzapine to haloperidol in efficacy (Leelahanaj et al., 2005). Both olanzapine and haloperidol were efficacious in the treatment of MAP, but olanzapine had lower frequency and severity of extrapyramidal symptoms. Zarrabi et al. evaluated the clinical course and treatment of 152 inpatient admissions and found that risperidone and olanzapine were used most frequently and that recovery from psychotic symptoms in 31.6% of the inpatients took more than 1 month (Zarrabi et al., 2016). These authors suggested that both risperidone and olanzapine are helpful with MAP symptoms but also help to restore weight and appetite, reduced by methamphetamine use. Another study conducted by Farnia and colleagues compared aripiprazole and risperidone and found that both were effective for patients with MAP (Farnia et al., 2016). These authors found that risperidone had the greater efficacy in reducing positive psychotic symptoms, but aripiprazole had a greater efficacy in reducing negative symptoms (Ashok et al., 2017; Lee et al., 2009; Volkow et al., 2001). Decreased D2 receptor availability has been associated with increased impulsivity (Lee et al., 2009) and pleasure (Volkow et al., 1999) from drug use in humans. In animal trials, rats with decreased D2 receptors demonstrate increased impulsivity and drug-seeking behavior (Dalley et al., 2007). These findings suggest that D2 receptor antagonists could exacerbate drug craving for methamphetamine. Since antipsychotic medications have an antagonistic effect on the D2 receptor, it is important to bear in mind the importance of administering the lowest effective dose of antipsychotic medication in patients with MAP to avoid exacerbating methamphetamine craving and use. Furthermore, paliperidone has demonstrated a faster dissociation rate from human-cloned D2 receptors in tissue culture compared to risperidone, chlorpromazine, haloperidol, and olanzapine (Seeman, 2005); in addition to decreased risk of extrapyramidal side effects, it could be postulated that medications with faster dissociation rate from D2 receptors may also minimize drug craving induced by D2 receptor antagonism; however, the evidence to support this claim is scarce thus far. Additionally, treating patients with MAP with the lowest effective dose is also of the utmost importance because of their increased risk of some adverse effects when treated with antipsychotic medications. Compared to patients with schizophrenia without methamphetamine use, patients with methamphetamine use disorder had 4 times higher odds of experiencing extrapyramidal side effects when receiving antipsychotic medication (Temmingh et al., 2020).
Regarding treatment compliance among patients with MAP, Asadi et al. found that patients with higher PANSS score had higher rates of noncompliance. Nonetheless, patients’ attitudes regarding treatment became more favorable over time (Asadi et al., 2021), which may increase treatment compliance.
Treatment of Chronic Map
The management of MAP, particularly treatment of refractory and persistent symptoms even after a period of abstinence, also remains ill-defined. Clinical guidelines lack an abundance of randomized controlled trials (Grigg et al., 2018). No antipsychotic is currently defined as superior to others in efficacy of reducing chronic methamphetamine-associated psychotic symptoms. Two recent systematic reviews conducted by Chiang et al. and Fluyau et al. in 2019 indicate that antipsychotics were effective for MAP (Chiang et al., 2019; Fluyau et al., 2019). Srisurapanont and colleagues conducted a systematic review and meta-analysis, and this data suggests that olanzapine and quetiapine are possibly superior to aripiprazole and risperidone in the treatment for MAP (Srisurapanont et al., 2021). However, the authors caution that these data should be interpreted with an abundance of caution due to the low quality of the trials and an overall paucity of evidence (Srisurapanont et al., 2021). Patients with MAP resistant to antipsychotics have also been described as responsive to clozapine treatment in case reports (Seddigh et al., 2014).
While antipsychotic medications may have limited efficacy in MAP, alternative treatments may be necessary for clinicians to consider. Case reports (Grelotti et al., 2010) and animal studies (YL et al., 2012) suggest that electroconvulsive therapy (ECT) may be beneficial in improving psychotic symptoms. However, an Iranian pilot study conducted on ten patients with chronic methamphetamine-associated psychotic symptoms found no difference in Brief Psychiatric Rating Scale scores in patients treated with ECT compared to those maintained on olanzapine (Ziaaddini et al., 2015). However, this study had a small sample size with only five patients in each group; larger longitudinal trials determining efficacy of ECT for MAP are needed.
Minocycline has also been studied recently for its efficacy in treating MAP. A small trial of five participants with treatment-resistant methamphetamine psychosis found that 200 mg minocycline once daily improved both positive and negative symptoms and neurocognitive function, such as auditory working memory, within 2 months (Alavi et al., 2021).
Long-term treatment of individuals with MAP should be tailored to the ongoing needs of patients as well as individuals’ risks for side effects. Management of MAP should also attend to reduction and, ideally, abstinence from methamphetamines to reduce a return or worsening of psychotic symptoms. Comprehensive treatment should be provided to address other co-occurring psychiatric symptoms as well as psychosocial interventions to bolster relapse prevention skills.
Conclusion
This article summarizes the current understanding of methamphetamine-associated psychosis, which is a significant and potentially long-lasting effect of increasing stimulant use worldwide. Its neurobiology, comparison to schizophrenia and other primary psychoses, and clinical and pharmacological treatment strategies summarize an adequate starting point for management; however, there is room for improvement in current standard of care. While some studies show promising results, additional longitudinal studies are needed to bolster evidence and provide more concrete data to inform clinical decision making. New pharmacological treatments and testing to differentiate between acute, chronic, and primary psychosis in people with MAP should be investigated to provide clinicians with additional tools in diagnosis and treatment.
References
Akiyama, K., Saito, A., & Shimoda, K. (2011). Chronic methamphetamine psychosis after long-term abstinence in Japanese incarcerated patients. The American Journal on Addictions, 20(3), 240–249. https://doi.org/10.1111/J.1521-0391.2011.00124.X
Alavi, S., Darharaj, M., Bilehsavar, S. H., Amini, M., Zafarghandi, M. B. S., Berenji, V., & Arezoomandan, R. (2021). Successful use of minocycline for the treatment of methamphetamine-induced psychosis and cognitive impairments: An open-label case series. Clinical Neuropharmacology, 44(4), 126–131. https://doi.org/10.1097/WNF.0000000000000460
American Psychiatric Association. (2022). Schizophrenia spectrum and other psychotic disorders. In Diagnostic and statistical manual of mental disorders (5th ed., text rev.). https://doi.org/10.1176/appi.books.9780890425787.x02_Schizophrenia_Spectrum
Angrist, B., Sathananthan, G., Wilk, S., & Gershon, S. (1974). Amphetamine psychosis: Behavioral and biochemical aspects. Journal of Psychiatric Research, 11(C), 13–23. https://doi.org/10.1016/0022-3956(74)90064-8
Arunogiri S., Foulds J. A., McKetin R., & Lubman D. I. (2018). A systematic review of risk factors for methamphetamine associated psychosis. Australian & New Zealand Journal of Psychiatry, 52(6), 514–529. https://doi.org/10.1177/0004867417748750
Asadi, M., Rashedi, V., Khademoreza, N., Seddigh, R., Keshavarz-Akhlaghi, A.-A., Ahmadkhaniha, H., Rezvaniardestani, S.-M., Lotfi, A., & Shalbafan, M. (2021). MD (2022) Medication adherence and drug attitude amongst male patients with the methamphetamine-induced psychotic disorder after discharge: A three months follow up study. Journal of Psychoactive Drugs, 54(1), 18–24. https://doi.org/10.1080/02791072.2021.1883778
Ashok, A. H., Mizuno, Y., Volkow, N. D., & Howes, O. D. (2017). Association of stimulant use with dopaminergic alterations in users of cocaine, amphetamine, or methamphetamine: A systematic review and meta-analysis. JAMA Psychiatry, 74(5), 511–519. https://doi.org/10.1001/JAMAPSYCHIATRY.2017.0135
Bahji, A. (2021). Methamphetamine-related emergency department visits requiring psychiatric admission: A retrospective cohort study. International Journal of Mental Health and Addiction, 19(4), 1362–1371. https://doi.org/10.1007/S11469-020-00230-2
Barr, A. M., Panenka, W. J., MacEwan, G. W., Thornton, A. E., Lang, D. J., Honer, W. G., & Lecomte, T. (2006). The need for speed: An update on methamphetamine addiction. Journal of Psychiatry and Neuroscience, 31(5), 301–313.
BELL, D. S. (1965). Comparison of amphetamine psychosis and schizophrenia. The British Journal of Psychiatry : The Journal of Mental Science, 111, 701–707. https://doi.org/10.1192/bjp.111.477.701
Bramness, J. G., Gundersen, Ø. H., Guterstam, J., et al. (2012). Amphetamine-induced psychosis — A separate diagnostic entity or primary psychosis triggered in the vulnerable? BMC Psychiatry, 12, 221. https://doi.org/10.1186/1471-244X-12-221
Breen, M. S., Uhlmann, A., Nday, C. M., Glatt, S. J., Mitt, M., Metsalpu, A., Stein, D. J., & Illing, N. (2016). Candidate gene networks and blood biomarkers of methamphetamine-associated psychosis: An integrative RNA-sequencing report. Translational Psychiatry, 6(5), 1802–1802. https://doi.org/10.1038/TP.2016.67
Chan, B., Freeman, M., Kondo, K., Ayers, C., Montgomery, J., Paynter, R., & Kansagara, D. (2019). Pharmacotherapy for methamphetamine/amphetamine use disorder—A systematic review and meta-analysis. Addiction, 114(12), 2122–2136. https://doi.org/10.1111/ADD.14755
Chen, C. K., Lin, S. K., Sham, P. C., Ball, D., Loh, E. W., Hsiao, C. C., Chiang, Y. L., Ree, S. C., Lee, C. H., & Murray, R. M. (2003). Pre-morbid characteristics and co-morbidity of methamphetamine users with and without psychosis. Psychological Medicine, 33(8), 1407–1414. https://doi.org/10.1017/S0033291703008353
Chen, C. K., Lin, S. K., Chen, Y. C., Huang, M. C., Chen, T. T., Ree, S. C., & Wang, L. J. (2015). Persistence of psychotic symptoms as an indicator of cognitive impairment in methamphetamine users. Drug and Alcohol Dependence, 148, 158–164. https://doi.org/10.1016/J.DRUGALCDEP.2014.12.035
Chiang, M., Lombardi, D., Du, J., Makrum, U., Sitthichai, R., Harrington, A., Shukair, N., Zhao, M., & Fan, X. (2019). Methamphetamine-associated psychosis: Clinical presentation, biological basis, and treatment options. Human Psychopharmacology, 34(5), e2710. https://doi.org/10.1002/hup.2710
Courtney, K. E., & Ray, L. A. (2014). Methamphetamine: An update on epidemiology, pharmacology, clinical phenomenology, and treatment literature. Drug and Alcohol Dependence, 1(143), 11–21. https://doi.org/10.1016/j.drugalcdep.2014.08.003
Cumming, C., Kinner, S. A., McKetin, R., Li, I., & Preen, D. (2020). Methamphetamine use, health and criminal justice system outcomes: A systematic review. Drug and Alcohol Review, 39(5), 505–518. https://doi.org/10.1111/dar.13062
Dahan, S., Bragazzi, N. L., Yogev, A., Bar-Gad, M., Barak, V., Amital, H., & Amital, D. (2018). The relationship between serum cytokine levels and degree of psychosis in patients with schizophrenia. Psychiatry Research, 268, 467–472. https://doi.org/10.1016/J.PSYCHRES.2018.07.041
Dalley, J. W., Fryer, T. D., Brichard, L., Robinson, E. S. J., Theobald, D. E. H., Lääne, K., Peña, Y., Murphy, E. R., Shah, Y., Probst, K., Abakumova, I., Aigbirhio, F. I., Richards, H. K., Hong, Y., Baron, J. C., Everitt, B. J., & Robbins, T. W. (2007). Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science, 315(5816), 1267–1270. https://doi.org/10.1126/SCIENCE.1137073
Ding, Y., Lin, H., Zhou, L., Yan, H., & He, N. (2014). Adverse childhood experiences and interaction with methamphetamine use frequency in the risk of methamphetamine-associated psychosis. Drug and Alcohol Dependence, 142, 295–300. https://doi.org/10.1016/J.DRUGALCDEP.2014.06.042
Elkashef, A., Kahn, R., Yu, E., Iturriaga, E., Li, S. H., Anderson, A., Chiang, N., Ait-Daoud, N., Weiss, D., Mcsherry, F., Serpi, T., Rawson, R., Hrymoc, M., Weis, D., Mccann, M., Pham, T., Stock, C., Dickinson, R., Campbell, J., …, Johnson, B. A. (2012). Topiramate for the treatment of methamphetamine addiction: A multi-center placebo-controlled trial. Addiction, 107(7), 1297–1306. https://doi.org/10.1111/J.1360-0443.2011.03771.X
Ezzatpanah, Z., Shariat, S. V., & Tehrani-Doost, M. (2014). Cognitive functions in methamphetamine induced psychosis compared to schizophrenia and normal subjects. Iranian Journal of Psychiatry, 9(3), 152–157.
Farnia, V., Shakeri, J., Tatari, F., Juibari, T. A., Bajoghli, H., Golshani, S., Hookari, S., Holsboer-Trachsler, E., & Brand, S. (2016). Demographic and mental history-related data predicted occurrence of psychosis in metamphetamine users. In Psychiatry Research, 240, 431–434. https://doi.org/10.1016/j.psychres.2016.04.053
Farnia, V., Farshchian, F., Farshchian, N., Alikhani, M., SadeghiBahmani, D., & Brand, S. (2020). Comparisons of voxel-based morphometric brain volumes of individuals with methamphetamine-induced psychotic disorder and schizophrenia spectrum disorder and healthy controls. Neuropsychobiology, 79(2), 170–178. https://doi.org/10.1159/000504576
Fasihpour, B., Molavi, S., & Shariat, S. V. (2013). Clinical features of inpatients with methamphetamine-induced psychosis. Journal of Mental Health, 22(4), 341–349. https://doi.org/10.3109/09638237.2012.745184
Fiorentini, A., Cantù, F., Crisanti, C., Cereda, G., Oldani, L., & Brambilla, P. (2021). Substance-induced psychoses: An updated literature review. Frontiers in Psychiatry, 12, 2270. https://doi.org/10.3389/FPSYT.2021.694863/BIBTEX
Fluyau, D., Mitra, P., & Lorthe, K. (2019). Antipsychotics for amphetamine psychosis. A systematic review. Frontiers in Psychiatry, 10, 740. https://doi.org/10.3389/fpsyt.2019.00740
Glasner-Edwards, S., & Mooney, L. J. (2014). Methamphetamine psychosis: Epidemiology and management. CNS Drugs, 28(12), 1115–1126. https://doi.org/10.1007/s40263-014-0209-8
Goldsmith, D. R., Rapaport, M. H., & Miller, B. J. (2016). A meta-analysis of blood cytokine network alterations in psychiatric patients: Comparisons between schizophrenia, bipolar disorder and depression. Molecular Psychiatry, 21(12), 1696–1709. https://doi.org/10.1038/mp.2016.3
Grelotti, D. J., Kanayama, G., & Pope, H. G. (2010). Remission of persistent methamphetamine-induced psychosis after electroconvulsive therapy: Presentation of a case and review of the literature. American Journal of Psychiatry, 167(1), 17–23. https://doi.org/10.1176/appi.ajp.2009.08111695
Grigg, J., Manning, V., Arunogiri, S., Volpe, I., Frei, M., Phan, V., Rubenis, A., Dias, S., Petrie, M., Sharkey, M., & Lubman, D. (2018). Methamphetamine treatment guidelines: practice guidelines for health professionals (2nd ed.) Turning Point.
Hajebi, A., Amini, H., Kashani, L., & Sharifi, V. (2018). Twelve-month course and outcome of methamphetamine-induced psychosis compared with first episode primary psychotic disorders. Early Intervention in Psychiatry, 12(5), 928–934. https://doi.org/10.1111/EIP.12404
Hsieh, J. H., Stein, D. J., & Howells, F. M. (2014). The neurobiology of methamphetamine induced psychosis. Frontiers in Human Neuroscience, 8, 537. https://doi.org/10.3389/fnhum.2014.00537
Iwanami, A., Sugiyama, A., Kuroki, N., Toda, S., Kato, N., Nakatani, Y., Horita, N., & Kaneko, T. (1994). Patients with methamphetamine psychosis admitted to a psychiatric hospital in Japan: A preliminary report. Acta Psychiatrica Scandinavica, 89(6), 428–432. https://doi.org/10.1111/j.1600-0447.1994.tb01541.x
Jacobs, E., Fujii, D., Schiffman, J., & Bello, I. (2008). An exploratory analysis of neurocognition in methamphetamine-induced psychotic disorder and paranoid schizophrenia. Cognitive and Behavioral Neurology, 21(2), 98–103. https://doi.org/10.1097/WNN.0b013e31816bdf90
Jan, R. K., Kydd, R. R., & Russell, B. R. (2012). Functional and structural brain changes associated with methamphetamine abuse. Brain Sciences, 2(4), 434–482. https://doi.org/10.3390/brainsci2040434
Jauhar, S., Johnstone, M., & McKenna, P. J. (2022). Schizophrenia. The Lancet, 399(10323), 473–486. https://doi.org/10.1016/s0140-6736(21)01730-x
Jayaram-Lindström, N., Hammarberg, A., Beck, O., & Franck, J. (2008). Naltrexone for the treatment of amphetamine dependence: A randomized, placebo-controlled trial. American Journal of Psychiatry, 165(11), 1442–1448. https://doi.org/10.1176/APPI.AJP.2008.08020304
Johnson, B., & Streltzer, J. (2013). Risks associated with long-term benzodiazepine use. American Family Physician, 88(4), 224.
Jones, A. A., Gicas, K. M., Seyedin, S., Willi, T. S., Leonova, O., Vila-Rodriguez, F., Procyshyn, R. M., Smith, G. N., Schmitt, T. A., Vertinsky, A. T., Buchanan, T., Rauscher, A., Lang, D. J., MacEwan, G. W., Lima, V. D., Montaner, J. S. G., Panenka, W. J., Barr, A. M., Thornton, A. E., & Honer, W. G. (2020). Associations of substance use, psychosis, and mortality among people living in precarious housing or homelessness: A longitudinal, community-based study in Vancouver, Canada. PLoS Medicine, 17(7), e1003172. https://doi.org/10.1371/journal.pmed.1003172
Kishimoto, M., Ujike, H., Motohashi, Y., Tanaka, Y., Okahisa, Y., Kotaka, T., Harano, M., Inada, T., Yamada, M., Komiyama, T., Hori, T., Sekine, Y., Iwata, N., Sora, I., Iyo, M., Ozaki, N., & Kuroda, S. (2008). The dysbindin gene (DTNBP1) is associated with methamphetamine psychosis. Biological Psychiatry, 63(2), 191–196. https://doi.org/10.1016/J.BIOPSYCH.2007.03.019
Konstenius, M., Jayaram-Lindström, N., Guterstam, J., Beck, O., Philips, B., & Franck, J. (2014). Methylphenidate for attention deficit hyperactivity disorder and drug relapse in criminal offenders with substance dependence: A 24-week randomized placebo-controlled trial. Addiction, 109(3), 440–449. https://doi.org/10.1111/ADD.12369
Kuitunen-Paul, S., Roessner, V., Basedow, L. A., & Golub, Y. (2020). Beyond the tip of the iceberg: A narrative review to identify research gaps on comorbid psychiatric disorders in adolescents with methamphetamine use disorder or chronic methamphetamine use. 42(1), 13–32. https://doi.org/10.1080/08897077.2020.1806183
Lapchak, P. A. (1992). A role for interleukin-2 in the regulation of striatal dopaminergic function. NeuroReport, 3(2), 165–168. https://doi.org/10.1097/00001756-199202000-00011
Lappin, J. M., & Sara, G. E. (2019). Psychostimulant use and the brain. Addiction (Abingdon, England), 114(11), 2065–2077. https://doi.org/10.1111/add.14708
Lee, B., London, E. D., Poldrack, R. A., Farahi, J., Nacca, A., Monterosso, J. R., Mumford, J. A., Bokarius, A. V., Dahlbom, M., Mukherjee, J., Bilder, R. M., Brody, A. L., & Mandelkern, M. A. (2009). Striatal dopamine D2/D3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. Journal of Neuroscience, 29(47), 14734–14740. https://doi.org/10.1523/JNEUROSCI.3765-09.2009
Leelahanaj, T., Kongsakon, R., & Netrakom, P. (2005). A 4-week, double-blind comparison of olanzapine with haloperidol in the treatment of amphetamine psychosis. Journal of the Medical Association of Thailand = Chotmaihet Thangphaet, 88 Suppl 3, S43-52.
Licinio, J., Seibyl, J. P., Altemus, M., Charney, D. S., & Krystal, J. H. (1993). Elevated CSF levels of interleukin-2 in neuroleptic-free schizophrenic patients. American Journal of Psychiatry, 150(9), 1408–1410. https://doi.org/10.1176/ajp.150.9.1408
Lineberry, T. W., & Bostwick, J. M. (2006). Methamphetamine abuse: A perfect storm of complications. Mayo Clinic Proceedings, 81(1), 77–84. https://doi.org/10.4065/81.1.77
McKetin, R., McLaren, J., Lubman, D. I., & Hides, L. (2006). The prevalence of psychotic symptoms among methamphetamine users. Addiction, 101(10), 1473–1478. https://doi.org/10.1111/J.1360-0443.2006.01496.X
McKetin, R., Hickey, K., Devlin, K., & Lawrence, K. (2010). The risk of psychotic symptoms associated with recreational methamphetamine use. Drug and Alcohol Review, 29(4), 358–363. https://doi.org/10.1111/J.1465-3362.2009.00160.X
McKetin, R., Lubman, D. I., Baker, A. L., & DaweS, Ali RL. (2013). Dose-related psychotic symptoms in chronic methamphetamine users: Evidence from a prospective longitudinal study. JAMA Psychiatry, 70(3), 319–324. https://doi.org/10.1001/JAMAPSYCHIATRY.2013.283
McKetin, R., Gardner, J., Baker, A. L., Dawe, S., Ali, R., Voce, A., Leach, L. S., & Lubman, D. I. (2016). Correlates of transient versus persistent psychotic symptoms among dependent methamphetamine users. Psychiatry Research, 238, 166–171. https://doi.org/10.1016/J.PSYCHRES.2016.02.038
Medhus, S., Mordal, J., Holm, B., Mørland, J., & Bramness, J. G. (2013). A comparison of symptoms and drug use between patients with methamphetamine associated psychoses and patients diagnosed with schizophrenia in two acute psychiatric wards. Psychiatry Research, 206(1), 17–21. https://doi.org/10.1016/J.PSYCHRES.2012.09.023
Naji, L., Dennis, B., Rosic, T., Wiercioch, W., Paul, J., Worster, A., Thabane, L., & Samaan, Z. (2022). Mirtazapine for the treatment of amphetamine and methamphetamine use disorder: A systematic review and meta-analysis. Drug and Alcohol Dependence, 232, 109295. https://doi.org/10.1016/J.DRUGALCDEP.2022.109295
Papageorgiou, M., Raza, A., Fraser, S., Nurgali, K., & Apostolopoulos, V. (2019). Methamphetamine and its immune-modulating effects. Maturitas, 121, 13–21. https://doi.org/10.1016/J.MATURITAS.2018.12.003
Paulus, M. P., & Stewart, J. L. (2020). Neurobiology, clinical presentation, and treatment of methamphetamine use disorder: A review. In JAMA Psychiatry, 77(9), 959–966. https://doi.org/10.1001/jamapsychiatry.2020.0246. American Medical Association.
Richards, J. R., Albertson, T. E., Derlet, R. W., Lange, R. A., Olson, K. R., & Horowitz, B. Z. (2015). Treatment of toxicity from amphetamines, related derivatives, and analogues: A systematic clinical review. Drug and Alcohol Dependence, 150, 1–13. https://doi.org/10.1016/J.DRUGALCDEP.2015.01.040
Ronsley, C., Nolan, S., Knight, R., Hayashi, K., Klimas, J., Walley, A., Wood, E., & Fairbairn, N. (2020). Treatment of stimulant use disorder: A systematic review of reviews. PLoS ONE, 15(6). https://doi.org/10.1371/JOURNAL.PONE.0234809
Seddigh, R., Keshavarz-Akhlaghi, A.-A., & Shariati, B. (2014). Treating methamphetamine-induced resistant psychosis with clozapine. Case Reports in Psychiatry, 2014, 845145. https://doi.org/10.1155/2014/845145
Seeman, P. (2005). An update of fast-off dopamine D2 atypical antipsychotics [4]. American Journal of Psychiatry, 162(10), 1984–1985. https://doi.org/10.1176/APPI.AJP.162.10.1984-A
Sekiguchi, Y., Okada, T., & Okumura, Y. (2021). Treatment response distinguishes persistent type of methamphetamine psychosis from schizophrenia spectrum disorder among inmates at Japanese medical prison. Frontiers in Psychiatry, 12, 1115. https://doi.org/10.3389/FPSYT.2021.629315/BIBTEX
Shelly, J., Uhlmann, A., Sinclair, H., Howells, F. M., Sibeko, G., Wilson, D., Stein, D. J., & Temmingh, H. (2016). First-rank symptoms in methamphetamine psychosis and schizophrenia. Psychopathology, 49(6), 429–435. https://doi.org/10.1159/000452476
Smith, M. J., Thirthalli, J., Abdallah, A. B., Murray, R. M., & Cottler, L. B. (2009). Prevalence of psychotic symptoms in substance users: A comparison across substances. Comprehensive Psychiatry, 50(3), 245. https://doi.org/10.1016/J.COMPPSYCH.2008.07.009
Srisurapanont, M., Arunpongpaisal, S., Wada, K., Marsden, J., Ali, R., & Kongsakon, R. (2011). Comparisons of methamphetamine psychotic and schizophrenic symptoms: A differential item functioning analysis. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 35(4), 959–964. https://doi.org/10.1016/J.PNPBP.2011.01.014
Srisurapanont, M., Likhitsathian, S., Suttajit, S., Maneeton, N., Maneeton, B., Oon‐arom, A., Suradom, C., & Oon-Arom, A. (2021). Efficacy and dropout rates of antipsychotic medications for methamphetamine psychosis: A systematic review and network meta-analysis. Drug & Alcohol Dependence, 219, 108467. https://doi.org/10.1016/j.drugalcdep.2020.108467
Su, M. F., Liu, M. X., Li, J. Q., Lappin, J. M., Li, S. X., Wu, P., Liu, Z. M., Shi, J., Lu, L., & Bao, Y. (2018). Epidemiological characteristics and risk factors of methamphetamine-associated psychotic symptoms. Frontiers in Psychiatry, 9(OCT), 489. https://doi.org/10.3389/FPSYT.2018.00489/BIBTEX
Substance Abuse and Mental Health Services Administration. (2023). Treatment episode data set admissions (TEDS-A) 2021: Public use file (PUF) codebook. Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. https://www.samhsa.gov/data/. Accessed 15 Nov 2023.
Sulaiman, A. H., Said, M. A., Habil, M. H., Rashid, R., Siddiq, A., Guan, N. C., Midin, M., Jaafar, N. R. N., Sidi, H., & Das, S. (2014). The risk and associated factors of methamphetamine psychosis in methamphetamine-dependent patients in Malaysia. Comprehensive Psychiatry, 55(SUPPL. 1), S89–S94. https://doi.org/10.1016/J.COMPPSYCH.2013.01.003
Temmingh, H. S., van den Brink, W., Howells, F., Sibeko, G., & Stein, D. J. (2020). Methamphetamine use and antipsychotic-related extrapyramidal side-effects in patients with psychotic disorders. Journal of Dual Diagnosis, 16(2), 208–217. https://doi.org/10.1080/15504263.2020.1714099
Teraoka, A. (1967). Prognostic and criminological studies on the mental disorder caused by the chronic methamphetamine intoxication. Seishin Shinkeigaku Zasshi = Psychiatria et Neurologia Japonica, 69(6), 597–619.
Verachai, V., Rukngan, W., Chawanakrasaesin, K., Nilaban, S., Suwanmajo, S., Thanateerabunjong, R., Kaewkungwal, J., & Kalayasiri, R. (2014). Treatment of methamphetamine-induced psychosis: A double-blind randomized controlled trial comparing haloperidol and quetiapine. Psychopharmacology (Berl), 231(16), 3099–3108. https://doi.org/10.1007/S00213-014-3485-6
Voce, A., Burns, R., Castle, D., Calabria, B., & McKetin, R. (2019a). Is there a discrete negative symptom syndrome in people who use methamphetamine? Comprehensive Psychiatry, 93, 27–32. https://doi.org/10.1016/J.COMPPSYCH.2019.06.002
Voce, A., Calabria, B., Burns, R., Castle, D., & McKetin, R. (2019b). A systematic review of the symptom profile and course of methamphetamine-associated psychosis: Substance use and misuse. Substance Use and Misuse, 54(4), 549–559. https://doi.org/10.1080/10826084.2018.1521430
Volkow, N. D., Wang, G. J., Fowler, J. S., Logan, J., Gatley, S. J., Gifford, A., Hitzemann, R., Ding, Y. S., & Pappas, N. (1999). Prediction of reinforcing responses to psychostimulants in humans by brain dopamine D2 receptor levels. American Journal of Psychiatry, 156(9), 1440–1443. https://doi.org/10.1176/ajp.156.9.1440
Volkow, N. D., Chang, L., Wang, G. J., Fowler, J. S., Ding, Y. S., Sedler, M., Logan, J., Franceschi, D., Gatley, J., Hitzemann, R., Gifford, A., Wong, C., & Pappas, N. (2001). Low level of brain dopamine D2 receptors in methamphetamine abusers: Association with metabolism in the orbitofrontal cortex. American Journal of Psychiatry, 158(12), 2015–2021. https://doi.org/10.1176/APPI.AJP.158.12.2015
Wang, L. J., Lin, S. K., Chen, Y. C., Huang, M. C., Chen, T. T., Ree, S. C., & Chen, C. K. (2016). Differences in clinical features of methamphetamine users with persistent psychosis and patients with schizophrenia. Psychopathology, 49(2), 108–115. https://doi.org/10.1159/000445065
Wang, G., Ding, F., Chawarski, M. C., Hao, W., Liu, X., Deng, Q., & Ouyang, X. (2020). Randomized controlled trial of paliperidone extended release versus risperidone for the treatment of methamphetamine-associated psychosis in Chinese patients. Frontiers in Psychiatry, 11, 237. https://doi.org/10.3389/FPSYT.2020.00237/BIBTEX
Wearne, T. A., & Cornish, J. L. (2018). A comparison of methamphetamine-induced psychosis and schizophrenia: A review of positive, negative, and cognitive symptomatology. Frontiers in Psychiatry, 9(OCT), 491. https://doi.org/10.3389/FPSYT.2018.00491/BIBTEX
Wodarz, N., Krampe-Scheidler, A., Christ, M., Fleischmann, H., Looser, W., Schoett, K., Vilsmeier, F., Bothe, L., Schaefer, C., & Gouzoulis- Mayfrank, E. (2017). Evidence-based guidelines for the pharmacological management of acute methamphetamine-related disorders and toxicity. Pharmacopsychiatry, 50(3), 87–95.
Yamamuro, K., Makinodan, M., Kimoto, S., Kishimoto, N., Morimoto, T., Toritsuka, M., Matsuoka, K., Takebayashi, Y., Takata, T., Takahashi, M., Tanimura, Y., Nishihata, Y., Matsuda, Y., Ota, T., Yoshino, H., Iida, J., & Kishimoto, T. (2015). Differential patterns of blood oxygenation in the prefrontal cortex between patients with methamphetamine-induced psychosis and schizophrenia. Scientific Reports, 5, 12107. https://doi.org/10.1038/SREP12107
Yang, M., Yang, C., Liu, T., & London, E. D. (2020a). Methamphetamine-associated psychosis: Links to drug use characteristics and similarity to primary psychosis. International Journal of Psychiatry in Clinical Practice, 24(1), 31–37. https://doi.org/10.1080/13651501.2019.1676451
Yang, X., Zhao, H., Liu, X., Xie, Q., Zhou, X., Deng, Q., & Wang, G. (2020b). The relationship between serum cytokine levels and the degree of psychosis and cognitive impairment in patients with methamphetamine-associated psychosis in Chinese patients. Frontiers in Psychiatry, 11, 1454. https://doi.org/10.3389/FPSYT.2020.594766/BIBTEX
Yl, C., Hh, C., & Ch, C. (2012). Effects of repeated electroconvulsive shock on methamphetamine-induced behavioral abnormalities in mice. Brain Stimulation, 5(3), 393–401. https://doi.org/10.1016/J.BRS.2011.04.004
Yui, K., Ikemoto, S., Ishiguro, T., & Goto, K. (2000). Studies of amphetamine or methamphetamine psychosis in Japan: Relation of methamphetamine psychosis to schizophrenia. Annals of the New York Academy of Sciences, 914, 1–12. https://doi.org/10.1111/J.1749-6632.2000.TB05178.X
Zarrabi, H., Khalkhali, M., Hamidi, A., Ahmadi, R., & Zavarmousavi, M. (2016). Clinical features, course and treatment of methamphetamine-induced psychosis in psychiatric inpatients. BMC Psychiatry, 16(1), 1–8. https://doi.org/10.1186/s12888-016-0745-5
Ziaaddini, H., Roohbakhsh, T., Nakhaee, N., & Ghaffari-Nejad, A. (2015). Effectiveness of electroconvulsive therapy in persistent methamphetamine psychosis: A pilot study. Addiction & Health, 7(1/2), 14.
Zweben, J. E., Cohen, J. B., Christian, D., Galloway, G. P., Salinardi, M., Parent, D., Iguchi, M. (2004). Psychiatric symptoms in methamphetamine users. The American Journal on Addictions, 13, 181–190. https://doi.org/10.1080/10550490490436055
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Disclaimer
The information presented does not represent the views of the US Air Force.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stacy, P., Frantz, J., Miller, G. et al. A Narrative Review of the Pathophysiology and Treatment of Methamphetamine-Associated Psychosis. Int J Ment Health Addiction (2024). https://doi.org/10.1007/s11469-024-01323-y
Accepted:
Published:
DOI: https://doi.org/10.1007/s11469-024-01323-y