Abstract
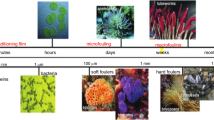
Adhesion of marine fouling organisms on artificial surfaces such as ship hulls causes many problems, including extra energy consumption, high maintenance costs, and increased corrosion. Therefore, marine antifouling is an important issue. In this review, physical and biochemical developments in the field of marine biofouling, which involves biofilm formation and macro-organism settlement, are discussed. The major antifouling technologies based on traditional chemical methods, biological methods, and physical methods are presented. The chemical methods include self-polishing types such as tributyltin (TBT) self-polishing copolymer coatings, which despite its good performance has been banned since 2008 because of its serious environmental impact. Therefore, other methods have been encouraged. These include coatings with copper compounds and biocide boosters to replace the TBT coatings. Biological extracts of secreted metabolites and enzymes are anticipated to act as antifoulants. Physical methods such as modification of surface topography, hydrophobic properties, and charge potential have also been considered to prevent biofouling. In this review, most of the current antifouling technologies are discussed. It is proposed that the physical antifouling technologies will be the ultimate antifouling solution, because of their broad-spectrum effectiveness and zero toxicity.
Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
References
Yebra D M, Kiil S, Dam J K. Antifouling technology — past, present and future steps towards efficient and environmentally friendly antifouling coatings. Prog Org Coat, 2004, 50: 75–104
Champ M. A review of organotin regulatory strategies, pending actions, related costs and benefits. Sci Total Environ, 2000, 258: 21–71
Abbott A, Abel P D, Arnold D W, et al. Cost-benefit analysis of the use of TBT: The case for a treatment approach. Sci Total Environ, 2000, 258: 5–19
Joseph J C, Ruey J T. Quantifying effects of antifouling paints on microbial biofilm formation. Methods Enzymol, 1999, 310: 637–645
Stefan M O. Controlled release of environmentally friendly antifouling agents from marine coatings. Dissertation for Doctoral Degree. Copenhagen: Technical University of Denmark, 2009
Maureen E C, Robert L F. The influence of low surface energy materials on bioadhesion — A review. Int Biodeterior Biodegrad, 1994, 34: 333–348
Fletcher M, Loeb G I. Influence of substratum characteristics on the attachment of a marine pseudomonad to solid surfaces. Appl Environ Microbiol, 1979, 37: 67–72
Walt D R, Smulow J B, Turesky S S, et al. The effect of gravity on initial microbial adhesion. J Colloid Interface Sci, 1985, 107: 334–336
Per R J, Kent M B, Ann I L. Linking larval supply to recruitment: Flow-mediated control of initial adhesion of barnacle larvae. Ecology, 2004, 85: 2850–2859
Chambers L D, Stokes K R, Walsh F C, et al. Modern approaches to marine antifouling coatings. Surf Coat Technol, 2006, 201: 3642–3652
Lewin R. Microbial adhesion is a sticky problem. Science, 1984, 224: 375–377
Abarzua S, Jakubowski S. Biotechnological investigation for the prevention of biofouling I. Biological and biochemical principles for the prevention of biofouling. Mar Ecol Prog Ser, 1995, 123: 301–312
Luciana V R, de Messano, Lucio S, et al. The effect of biofouling on localized corrosion of the stainless steels N08904 and UNS S32760. Int Biodeterior Biodegrad, 2009, 63: 607–614
Cooksey K E, Wigglesworth B C. Adhesion of bacteria and diatoms to surfaces in the sea: A review. Aquat Microb Ecol, 1995, 9: 87–96
Maki J S, Rittschof D, Schmidt A R, et al. Factors controlling adhesion of bryozoan larvae: A comparison of bacterial films and unfilmed surfaces. Biol Bull, 1989, 177: 295–302
Lau S C K, Harder T, Qian P Y. Induction of larval settlement in the serpulid polychaete Hydroides elegans (Haswell): Role of bacterial extracellular polymers. Biofouling, 2003, 19: 197–204
Hung O S, Thiyagarajan V, Wu R S S, et al. Effects of ultraviolet radiation on films and subsequent settlement of Hydroides elegans. Mar Ecol Prog Ser, 2005, 304: 155–166
Lasa I. Towards the identification of the common features of bacterial biofilm development. Int Microbiol, 2006, 9: 21–28
Flemming H C, Griebe T, Schaule G. Antifouling strategies in technical systems—a short review. Water Sci Technol, 1996, 34: 517–524
Costerton J W. Overview of microbial biofilms. J Ind Microbiol, 1995, 15: 137–140
Stoodley P, Sauer K, Davies D G, et al. Biofilms as complex differentiated communities. Annu Rev Microbiol, 2002, 56: 187–209
Waters C M, Bassler B L. Quorum sensing: Cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol, 2005, 21: 319–346
Krug P. Defence of benthic invertebrates against surface colonization by larvae: A chemical arms race. Prog Mol Subcell Biol, 2006, 42: 1–53
Jayaraman M, Seetharaman J. Physicochemical analyses of the exopolysaccharides produced by a marine biofouling bacterium, Vibrio alginolyticus. Process Biochem, 2003, 38: 841–847
Jakob B K, Rikke L M, Brian S L, et al. Antifouling enzymes and the biochemistry of marine settlement. Biotechnol Adv, 2008, 26: 471–481
Latasa. Biofilm-associated proteins. C R Biologies, 2006, 329: 849–857
Larsen P, Nielsen J L, Dueholm M S, et al. Amyloid adhesins are abundant in natural biofilms. Environ Microbiol, 2007, 9: 3077–3090
Yanming X, Keiichi H. Amyloid fibril proteins. Mech Ageing Dev, 2002, 123: 1625–1636
Wetzel R, Shivaprasad S, Williams A D. Plasticity of amyloid fibrils. Biochemistry, 2007, 46: 1–10
Kiorboe T. Turbulence, phytoplankton cell size, and the structure of pelagic food webs. Adv Mar Biol, 1993, 29: 1–72
Finlay J A, Callow M E, Ista L K, et al. Adhesion strength of settled spores of the green alga enteromorpha and the diatom amphora. Integr Comp Biol, 2002, 42: 1116–1122
Sitaraman K, Nick W, Christopher K O, et al. Comparison of the fouling release properties of hydrophobic fluorinated and hydrophilic pegylated block copolymer surfaces: Attachment strength of the diatom navicula and the green alga ulva. Biomacromolecules, 2006, 7: 1449–1462
Jeffery R S, Sherilyn C F. Three-dimensional modeling of lacustrine diatom habitat areas: Improving paleolimnological interpretation of planktic: Benthic ratios. Limnol Oceanogr, 2004, 49: 1540–1548
Gross F, Zeuthen E. The buoyancy of plankton diatoms: A problem of cell physiology. Proc R Soc Lond Ser B, 1948, 135: 382–389
Ille C G, Herbert S, Manfred D. Diatom bionanotribology—biological surfaces in relative motion: Their design, friction, adhesion, lubrication and wear. J Nanosci Nanotechno, 2005, 5: 1–9
Kellar A, Yiching A L, Tonia S H, et al. Adhesive force of a single gecko foot-hair. Nature, 2000, 405: 681–685
Holland R, Dugdale T, Wetherbee R, et al. Adhesion and motility of fouling diatoms on a silicone elastomer. Biofouling, 2004, 20: 323–329
Edgar L A, Zavortink M. The mechanism of diatom locomotion. II. Identification of actin. Proc R Soc London Ser B, 1983, 218: 345–348
Nicole C P, Ilan S, Timothy P S, et al. Diatom gliding is the result of an actin-myosin motility system. Cell Motil Cytoskel, 1999, 44: 23–33
Lind J L, Heimann K, Miller E A, et al. Substratum adhesion and gliding in a diatom are mediated by extracellular proteoglycans. Planta, 1997, 203: 213–221
Gordon R, Drum R W. A Capillarity mechanism for diatom gliding locomotion. Proc Natl Acad Sci USA, 1970, 67: 338–344
Edgar L A. Diatom locomotion: A consideration of movement in a highly viscous situation. Eur J Phycol, 1982, 17: 243–251
Edgar L A, Pickett H J D. The mechanism of diatom locomotion. i. an ultrastructural study of the motility apparatus. Proc R Soc Lond Ser B, 1983, 218: 331–343
Wetherbee R, Lind J L, Burke J, et al. The first kiss: Establishment and control of initial adhesion by raphid diatoms. J Phycol, 1998, 34: 9–15
Chiovitti A, Dugdale T M, Wetherbee R. Diatom adhesives: Molecular and mechanical properties. In: Smith A M, Callow J A, eds. Biological Adhesives. Berlin Heidelberg: Springer-Verlag, 2006
Bhaskar P V, Narayan B B. Microbial extracellular polymeric substances in marine biogeochemical processes. Curr Sci, 2005, 88: 45–53
Rahul A B, PETER G K. Localization of EPS components secreted by freshwater diatoms using differential staining with fluorophore-conjugated lectins and other fluorochromes. Eur J Phycol, 2007, 42: 199–208
Yan W, Ya C, Colleen L, et al. Extracellular matrix assembly in diatoms (Bacillariophyceae). IV. Ultrastructure of Achnanthes longipes and Cymbella cistula as revealed by high-pressure freezing/freeze substitution and cryo-field emission scanning electron microscopy. J Phycol, 2000, 36: 367–378
Wustman B A, Gretz M R, Hoagland K D. Extracellular matrix assembly in diatoms (Bacillariophyceae) —1. A model of adhesives based on chemical characterization and localization of polysaccharides from the marine diatom Achnanthes longipes and other diatoms. Plant Physiol, 1997, 113: 1059–1069
Rakhee D S K, Narayan B B. Extracellular polymeric substances of the marine fouling diatom amphora rostrata Wm.Sm. Biofouling, 2001, 17: 117–127
Michael J H, Simon A C, Paul M, et al. Characterization of the adhesive mucilages secreted by live diatom cells using atomic force microscopy. Protist, 2002, 153: 25–38
Michael J H, Paul M, Paul M U, et al. The structure and nanome-chanical properties of the adhesive mucilage that mediates diatom-substratum adhesion and motility. J Phycol, 2003, 39: 1181–1193
de Brouwer J F C, Cooksey K E, Wigglesworth C B, et al. Time of flight-secondary ion mass spectrometry on isolated extracellular fractions and intact biofilms of three species of benthic diatoms. J Microbiol Methods, 2006, 65: 562–572
Tony M D, Anusuya W, Wetherbee R. Adhesive modular proteins occur in the extracellular mucilage of the motile, pennate diatom phaeodactylum tricornutum. Biophys J, 2006, 90: 58–60
John M. A Comparison of the value of various flagellates and diatoms as food for barnacle larvae. ICES J Mar Sci, 1963: 175-187
Huang S, Hadfield M G. Composition and density of bacterial biofilms determine larval settlement of the polychaete hydroides elegans. Mar Ecol Prog Ser, 2003, 260: 161–172
Patil J S, Anil A C. Influence of diatom exopolymers and biofilms on metamorphosis in the barnacle Balanus amphitrite. Mar Ecol Prog Ser, 2005, 301: 231–245
Dobretsov S, Xiong H, Xu Y, et al. Novel antifoulants: Inhibition of larval adhesion by proteases. Mar Biotechnol, 2007, 9: 388–397
Nobuhiro F. Biofouling and antifouling. Nat Prod Rep, 2004, 21: 94–104
Lagersson N, Høeg J. Settlement behavior and antennary biomechanics in cypris larvae of Balanus amphitrite (Crustacea: Thecostraca: Cirripedia). Mar Biol, 2002, 141: 513–526
Kristin Ö, Christian A, James T R, et al. An in vivo study of exocytosis of cement proteins from barnacle Balanus improvisus (D.) cyprid larva. J Exp Biol, 2006, 209: 956–964
Callow J A, Crawford S, Higgins M, et al. The application of atomic force microscopy to topographical studies and force measurements on the secreted adhesive of the green alga Enteromorpha. Planta, 2000, 211: 641–647
Callow M E, Callow J A, Pickett H J D, et al. Primary adhesion of Enteromorpha (chlorophyta, Ulvales) propagules: Quantitative settlement studies and video microscopy. J Phycol, 1997, 33: 938–947
Rosenhahn A, Finlay J A, Pettit M E, et al. Zeta potential of motile spores of the green alga Ulva linza and the influence of electrostatic interactions on spore settlement and adhesion strength. Biointerphases, 2009, 4: 7–11
Ederth T, Nygren P, Pettitt M E, et al. Anomalous settlement behavior of Ulva linza zoospores on cationic oligopeptide surfaces. Biofouling, 2008, 24: 303–312
Finlay J A, Callow M E, Schultz M P, et al. Adhesion strength of settled spores of the green alga Enteromorpha. Biofouling, 2002, 18: 251–256
Callow J A, Stanley M S, Wetherbee R, et al. Cellular and molecular approaches to understanding primary adhesion in Enteromorpha: An overview. Biofouling, 2000, 16: 141–150
Kamino K, Koji I, Tadashi M, et al. Barnacle cement proteins — importance of disulfide bonds in their insolubility. J Biol Chem, 2000, 275: 27360–27365
Kamino K. Novel barnacle underwater adhesive protein is a charged amino acid-rich protein constituted by a Cys-rich repetitive sequence. Biochem J, 2001, 356: 503–507
Admiraal W. Influence of light and temperature on the growth rate of estuarine benthic diatoms in culture. Mar Biol, 1997, 39: 1–9
Rascio V J D. Antifouling coatings: Where do we go from here. Corros Rev, 2003, 18: 133–154
Peter D S, Schneider R, Staffan K. Chemical defenses of seaweeds against microbial colonization. Biodegradation, 1997, 8: 211–220
Callow M E. Ship fouling: Problems and solutions. Chem Ind,1990, 5: 123–127
Iwao O. Organotin antifouling paints and their alternatives. Appl Organomet Chem, 2003, 17: 81–105
Marson F. Antifouling paints. I. Theoretical approach to leaching of soluble pigments from insoluble paint vehicles. J Appl Chem, 1969, 19: 93–99
Rascio V, Giúdice C, Amo B D. High-build soluble matrix antifouling paints tested on raft and ship’s bottom. Prog Org Coat, 1990, 18: 389–398
Yebra D M, Kiil S, Claus E W, et al. Dissolution rate measurements of sea water soluble pigments for antifouling paints: ZnO. Prog Org Coat, 2006, 56: 327–337
Kiil S, Claus E W, Michael S P, et al. Analysis of self-polishing antifouling paints using rotary experiments and mathematical modeling. Ind Eng Chem Res, 2001, 40: 3906–3920
Yebra D M, Kiil S, Claus E W, et al. Effects of marine microbial biofilms on the biocide release rate from antifouling paints—model-based analysis. Prog Org Coat, 2006, 57: 56–66
Anna K. Environmental management aspects for TBT antifouling wastes from the shipyards. J Environ Manage, 2009, 90(S): 77–85
Wisniewski N, Reichert M. Methods for reducing biosensor membrane biofouling. Colloids Surf B, 2000, 18: 197–219
Comber S D W, Franklin G, Gardner M J, et al. Partitioning of marine antifoulants in the marine environment. Sci Total Environ, 2002, 286: 61–71
Iwao O. General Aspects of tin-free antifouling paints. Chem Rev, 2003, 103: 3431–3448
Voulvoulis N, Scrimshaw M D, Lester J N. Alternative antifouling biocides. Appl Organomet Chem, 1999, 13: 135–143
Anita G J B, Sascha B S, Willem H P, et al. Impact of the antifouling agent Irgarol 1051 on marine phytoplankton species. J Sea Res, 2009, 61: 133–139
Shtykova L, Fant C, Handa P, et al. Adsorption of antifouling booster biocides on metal oxide nanoparticles: Effect of different metal oxides and solvents. Prog Org Coat, 2009, 64: 20–26
Wang J L, Wang F Q, Yu J, et al. A survey analysis of heavy metals bio-accumulation in internal organs of sea shell animals affected by the sustainable pollution of antifouling paints used for ships anchored at some domestic maritime spaces. Chinese Sci Bull, 2008, 53: 2471–2475
Loschau M, Kratke R. Efficacy and toxicity of self-polishing biocide-free antifouling paints. Environ Pollut, 2005, 138: 260–267
Chen M L, Qu Y Y, Yang L, et al. Structures and antifouling properties of low surface energy non-toxic antifouling coatings modified by nano-SiO2 powder. Sci China Ser B: Chem, 2008, 51: 848–852
Brady R F. A fracture mechanical analysis of fouling release from nontoxic antifouling coatings. Prog Org Coat, 2001, 43: 188–192
Umemura K, Yamada T, Maeda Y, et al. Regulated growth of diatom cells on self-assembled monolayers. J Nanobiotech, 2007, 5: 2–15
Abarzua S, Jakubowski S, Eckert S, et al. Biotechnological investigation for the prevention of marine biofouling II. Blue-green algae as potential producers of biogenic agents for the growth inhibition of microfouling organisms. Bot Mar, 1999, 42: 459–465
Xiong H R, Qi S H, Xu Y, et al. Antibiotic and antifouling compound production by the marine-derived fungus Cladosporium sp. F14. J Hydro-environ Res, 2009, 2: 264–270
Limna M V P, Raveendran T V, Parameswaran P S. Antifouling activity exhibited by secondary metabolites of the marine sponge, Haliclona exigua (Kirkpatrick). Int Biodeterior Biodegrad, 2009, 63: 67–72
Fernando S A, Carlos R. Inhibition of attachment of some fouling diatoms and settlement of Ulva lactuca zoospores by film-forming bacterium and their extracellular products isolated from biofouled substrata in Northern Chile. Electron J Biotechnol, 2008, 11: 1–11
Burgess J G, Boyd K G, Armstrong E, et al. The development of a marine natural product-based antifouling paint. Biofouling, 2003, 19(S): 197–205
Moss G. Enzyme nomenclature-recommendations of the nomenclature committee of the international union of biochemistry and molecular biology on the nomenclatureand classification of enzymes by the reactions they catalyse. Online edition. International Union of Biochemistry and Molecular Biology (NC-IUBMB). Tipton K F, Boyce S, eds. Department of Chemistry, Queen Mary University of London, Mile End Road, London, E1 4NS, UK. http://www.chem.qmul.ac.uk/iubmb/enzyme/index.html. 2006. Accessed 01-27-2008
Pettitt M E, Henry S L, Callow M E, et al. Activity of commercial enzymes on settlement and adhesion of cypris larvae of the barnacle balanus amphitrite, spores of the green alga Ulva linza, and the diatom Navicula perminuta. Biofouling, 2004, 20: 299–311
Charlotte J, Falholt P, Gram L. Enzymatic removal and disinfection of bacterial biofilms. Appl Environ Microbiol, 1997, 63: 3724–3728
Asuri P, Sandeep S K, Ravi S K, et al. Polymer-nanotube-enzyme composites as active antifouling films. Small, 2007, 3: 50–53
Kim Y D, Jonathan S D, Douglas S C. Siloxane-based biocatalytic films and paints for use as reactive coatings. Biotechnol Bioeng, 2001, 72: 475–482
Novick S J, Jonathan S D. Protein-containing hydrophobic coatings and films. Biomaterials, 2002, 23: 441–448
Leroy C, Delbarre C, Ghillebaert F, et al. Effects of commercial enzymes on the adhesion of a marine biofilm-forming bacterium. Biofouling, 2008, 24: 11–22
Luckarift H R, Matthew B D, Kenneth H S, et al. Room-temperature synthesis of antibacterial bionanocomposites of lysozyme with amorphous silica or titania. Small, 2006, 2: 640–643
Nick A, Phang I Y, Conlan S L, et al. The effects of a serine protease, Alcalase, on the adhesives of barnacle cyprids (Balanus amphitrite). Biofouling, 2008, 24: 97–107
Chiovitti A, Higgins M J, Harper R E, et al. The complex polysaccharides of the raphid diatom Pinnularia viridis (Bacillariophyceae). J Phycol, 2003, 39: 543–54
Joao B X, Cristian P, Suriani A R, et al. Biofilm-control strategies based on enzymic disruption of the extracellular polymeric substance matrix—A modelling study. Microbiology, 2005, 151: 3817–3832
Boyd A, Chakrabarty A M. Role of alginate lyase in cell detachment of Pseudomonas aeruginosa. Appl Environ Microbiol, 1994, 60: 2355–2359
Imlay J A. Pathways of oxidative damage. Annu Rev Microbiol, 2003, 57: 395–418
Callow J A, Callow M E. Biofilms. Prog Mol Subcell Biol, 2006, 42: 141–169
Huang Y L, Dobretsov S, Jang S K, et al. Presence of acylhomoserine lactone in subtidal biofilm and the implication in larval behavioral response in the polychaete hydroides elegans. Microb Ecol, 2008, 54: 384–392
Olsen S M, Pedersen L T, Laursen M H, et al. Enzyme-based antifouling coatings: A review. Biofouling, 2007, 23: 369–383
Chiang W C, Chyou S D, Huang R, et al. Control of marine biofouling by conductive coatings. Corros Prevent Control, 2000, 47: 121–128
Tadashi M, Tae K L. Electrochemical prevention of biofouling. Electrochemistry, 2000, 68: 847–852
Liang C H, Huang N B. Research on electrochemical behavior of titanium-supported anodic coating in electrolytic anti-fouling of brine. Mater Chem Phys, 2008, 111: 244–248
Sanford E B, Rittscho D. An investigation of low frequency sound waves as a means of inhibiting barnacle settlement. J Exp Mar Biol Ecol, 1984, 79: 149–154
Miloud R, Mireille L. Application of mechanical waves induced by piezofilms to marine fouling protection of oceanographic sensors. Smart Mater Struct, 1995, 4: 195–201
Finlay J A, Fletcher B R, Callow M E, et al. Effect of background colour on growth and adhesion strength of Ulva sporelings. Biofouling, 24: 219–225
Swain G, Herpe S, Ralson E, et al. Short-term testing of antifouling surfaces: The importance of colour. Biofouling, 2006, 22: 425–429
Bowen J, Pettitt M E, Kendall K, et al. The influence of surface lubricity on the adhesion of Navicula perminuta and Ulva linza to alkanethiolself-assembled monolayers. J R Soc Interface, 2007, 4: 473–477
Ista L, Callow M, Finlay J, et al. Effect of substratum surface chemistry and surface energy on adhesion of marine bacteria and algal spores. Appl Environ Microbiol, 2004, 70: 4151–4157
Mark P, Paul T, Sara S, et al. Effects of ultrafiltration membrane surface properties on Pseudomonas aeruginosa biofilm initiation for the purpose of reducing biofouling. J Membrane Sci, 2001, 194: 15–32
Callow M E, Callow J A, Ista L K, et al. The use of self-assembled monolayers (SAMs) of different wettability to study surface selection and primary adhesion processes of zoospores of the green alga Enteromorpha. Appl Environ Microbiol, 2000, 66: 3249–3254
Statz A, Finlay J, Dalsin J, et al. Algal antifouling and fouling-release properties of metal surfaces coated with a polymer inspired by marine mussels. Biofouling, 2006, 22: 391–399
Schilp S, Kueller A, Rosenhahn A, et al. Settlement and adhesion of algal cells to hexa(ethylene glycol)-containing self-assembled monolayers with systematically changed wetting properties. Biointerphases, 2007, 2: 143–150
Dineshrama R, Subasrib R, Somarajub K R C, et al. Biofouling studies on nanoparticle-based metal oxide coatings on glass coupons exposed to marine environment. Colloids Surf B, 2009, 74: 75–83
Finlay J A, Krishnan S, Callow M E, et al. Settlement of Ulva zoospores on patterned fluorinated and PEG-lated monolayer surfaces. Langmuir, 2008, 24: 503–510
Grozea C M, Gunari N, Finlay J A, et al. Water-stable diblock polystyrene-block-poly(2-vinyl pyridine) and diblock polystyrene-block-poly(methyl methacrylate) cylindrical patterned surfaces inhibit settlement of zoospores of the green alga ulva. Biomacromolecules, 2009, 10: 1004–1012
Scardino A J, Harvey E R, de Nys R. Testing adhesion point theory: diatom adhesion on microtextured polyimide biomimics. Biofouling, 2006, 22: 55–60
Scardino A J, Guenther J, de Nys R. Attachment point theory revisited: The fouling response to a microtextured matrix. Biofouling, 2008, 24: 45–53
Berntsson K M, Andreasson H, Jonsson P R, et al. Reduction of barnacle recruitment on micro-textured surfaces: Analysis of effective topographic characteristics and evaluation of skin friction. Biofouling, 2000, 16: 245–261
Schumacher J F, Carmen M L, Estes T G, et al. Engineered antifouling microtopographies—Effect of feature size, geometry, and roughness on settlement of zoospores of the green alga Ulva. Biofouling, 2007, 23: 55–62
Carmen M L, Estes T G, Feinberg A W, et al. Engineered antifouling microtopographies—Correlating wettability with cell attachment. Biofouling, 2006, 22: 11–21
Chung K K, Schumacher J F, Sampson E M, et al. Impact of engineered surface microtopography on biofilm formation of Staphylococcus aureus. Biointerphases, 2007, 2: 89–94
Knoell T, Safarik J, Cormack T, et al. Biofouling potentials of microporous polysulfone membranes containing a sulfonated polyether-ethersulfone/polyethersulfone block copolymer: Correlation of membrane surface properties with bacterial attachment. J Membr Sci, 1999, 157: 117–138
Busscher H J, van de Belt-Gritter B, van der Mei H C. Implications of microbial adhesion to hydrocarbons for evaluating cell surface hydrophobicity 1. Zeta potentials of hydrocarbon droplets. Colloids Surf B, 1995, 5: 111–116
Wilson W W, Wade M M, Holman S C, et al. Status of methods for assessing bacterial cell surface charge properties based on zeta potential measurements. J Microbiol Methods, 2001, 43: 153–164
Kamlesh A S, Ashwin K B, Ali B, et al. Zeta potential of selected bacteria in drinking water when dead, starved, or exposed to minimal and rich culture media. Curr Microbiol, 2008, 56: 93–97
Rita K H, Andy B, Simon A P, et al. Characterisation of algogenic organic matter extracted from cyanobacteria, green algae and diatoms. Water Res, 2008, 42: 3435–3445
Dittrich M, Sibler S. Cell surface groups of two picocyanobacteria strains studied by zeta potential investigations, potentiometric titration, and infrared spectroscopy. J Colloid Interface Sci, 2005, 286: 487–495
Maria O P, Maria J V, Vitorino M B, et al. Retention of bacteria by cellulose fibers as a means of reducing biofouling in paper pulp production process. Biofouling, 1998, 13: 1–18
Herrwerth S, Eck W, Reinhardt S, et al. Factors that determine the protein resistance of oligoether self-assembled monolayers—Internal hydrophilicity, terminal hydrophilicity, and lateral packing density. J Am Chem Soc, 2003, 125: 9359–9366
Soeren S, Alexander K, Axel R, et al. Settlement and adhesion of algal cells to hexa (ethylene glycol)-containing self-assembled monolayers with systematically changed wetting properties. Biointerphases, 2007, 2: 143–150
Jurgen K H, Richard L C W, Michael G. Hydroxide ion adsorption on self-assembled monolayers. J Am Chem Soc, 2003, 125: 8384–8389
Jansen B, Kohnen W. Prevention of biofilm formation by polymer modification. J Ind Microbiol, 1995, 15: 391–396
Gross M, Carmton S E, Gotz F, et al. Key role of teichoic acid net charge in staphylococcus aureus colonization of artificial surfaces. Infec Immunity, 2001, 69: 3423–3426
Schumacher J F, Long C J, Callow M E, et al. Engineered nanoforce gradients for inhibition of settlement (attachment) of swimming algal spores. Langmuir, 2008, 24: 4931–4937
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is published with open access at Springerlink.com
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Cao, S., Wang, J., Chen, H. et al. Progress of marine biofouling and antifouling technologies. Chin. Sci. Bull. 56, 598–612 (2011). https://doi.org/10.1007/s11434-010-4158-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11434-010-4158-4