Abstract
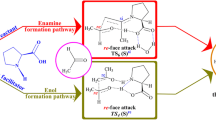
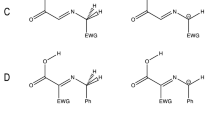
The role of water and stereoselectivity in the direct syn-aldol reaction involving 3-pentanone and 4-nitrobenzaldehyde catalyzed by amino acid derivatives on water has been investigated by density functional theory. Calculations indicate that the formation of intermediate enamine is the rate determining step via a three-step process with activation enthalpies of 50 kcal/mol in the gas phase and 21 kcal/mol in the presence of water. The subsequent nucleophilic addition of enamine to aldehyde is relatively easier with activation enthalpies below 10 kcal/mol both in the gas phase and in the presence of water. The diastereoselective formation of syn- and anti-aldol products results from the preferential formation of Z-enamine to E-enamine, kinetically and thermodynamically. The enantioselectivity of both syn- and anti-products is controlled by the steric repulsive interactions between the amino alcohol moiety of catalyst and the phenyl ring of aldehyde. Calculations show that water molecule can act as a proton shuttle in the proton-transport catalytic processes. The water-assisted proton-transfer is very efficient to reduce the activation barriers via protonation and deprotonation in the formation of C-N and C-C bonds, dehydration, and β-elimination processes by inhibiting the generation of zwitterionic transition states. The theoretical discoveries indicate that in the present proton-transport assistance, the amino alcohol moiety of the catalyst plays a critical role as hydrogen bond donor to anchor substrates with carbonyl group close to the amine or enamine moiety so that the water molecule can bridge the NH of amine and the oxygen of carbonyl by hydrogen bonding interaction around the reactive site to activate the reactants and promote the reaction effectively.
Similar content being viewed by others
References
List B, Lerner R A, Barbas C F. Proline-catalyzed direct asymmetric aldol reactions. J Am Chem Soc, 2000, 122: 2395–2396
Mukherjee S, Yang Y W, Hoffmann S, et al. Asymmetric enamine catalysis. Chem Rev, 2007, 107: 5471–5569
Dondoni A, Massi A. Asymmetric organocatalysis: From infancy to adolescence. Angew Chem Int Ed, 2008, 47: 4638–4660
Melchiorre P, Marigo M, Carlone A, et al. Asymmetric aminocatalysis-Gold rush in organic chemistry. Angew Chem Int Ed, 2008, 47: 6138–6171
Li C J. Organic reactions in aqueous media with a focus on carbon carbon bond formations: A decade update. Chem Rev, 2005, 105: 3095–3165
Mlynarski J, Paradowska J. Catalytic asymmetric aldol reactions in aqueous media. Chem Soc Rev, 2008, 37: 1502–1511
Chanda A, Forkin V V. Organic synthesis “on water”. Chem Rev, 2009, 109: 725–748
Paradowska J, Stodulski M, Mlynarski J. Catalysts based on amino acids for asymmetric reactions in water. Angew Chem Int Ed, 2009, 48: 2–18
Dickerson T J, Janda K D. Aqueous aldol catalysis by a nicotine metabolite. J Am Chem Soc, 2002, 124: 3220–3221
Torii H, Nakadai M, Ishihara K, et al. Asymmetric direct aldol reaction assisted by water and a proline-derived tetrazole catalyst. Angew Chem Int Ed, 2004, 43: 1983–1986
Nyberg A I, Usano A, Pihko P M. Proline-catalyzed ketone-aldehyde aldol reactions are accelerated by water. Synlett, 2004: 1891–1896
Tang Z, Yang Z H, Cun L F, et al. Small peptides catalyze highly enantioselective direct aldol reactions of aldehydes with hydroxyacetone: Unprecedented regiocontrol in aqueous media. Org Lett, 2004, 6: 2285–2287
Mase N, Nakai Y, Ohara N, et al. Organocatalytic direct asymmetric aldol reactions in water. J Am Chem Soc, 2006, 128: 734–735
Hayashi Y, Sumiya T, Takahashi J, et al. Highly diastereo- and enantioselective direct aldol reactions in water. Angew Chem Int Ed, 2006, 45: 958–961
Hayashi Y, Aratake S, Okano T, et al. Combined proline-surfactant organocatalyst for the highly diastereo- and enantioselective aqueous direct cross-aldol reaction of aldehydes. Angew Chem Int Ed, 2006, 45: 5527–5529
Huang J, Zhang X, Armstrong D W. Highly efficient asymmetric direct stoichiometric aldol reactions on/in water. Angew Chem Int Ed, 2007, 46: 9073–9077
Zotova N, Franzke A, Armstrong A, et al. Clarification of the role of water in proline-mediated aldol reactions. J Am Chem Soc, 2007, 129: 15100–15101
Hayashi Y, Aratake S, Itoh T, et al. Dry and wet proline for asymmetric organic solvent-free aldehyde-aldehyde and aldehydeketone aldol reactions. Chem Commun, 2007, 957-959
Blackmond D G, Armstrong A, Coombe V, et al. Water in organocatalytic process: Debunking the myths. Angew Chem Int Ed, 2007, 46: 3798–3800
Jung Y, Marcus R A. On the theory of organic catalysis “on water”. J Am Chem Soc, 2007, 129: 5492–5502
Clemente F R, Houk K N. Computational evidence for the enamine mechanism of intramolecular aldol reactions catalyzed by proline. Angew Chem Int Ed, 2004, 43: 5766–5768
Rankin K N, Gauld J W, Boyd R J. Density functional study of the proline-catalyzed direct aldol reaction. J Phys Chem A, 2002, 106: 5155–5159
Tang Z, Jiang F, Cui X, et al. Enantioselective direct aldol reactions catalyzed by L-prolinamide derivatives. Proc Natl Acad Sci USA, 2004, 101: 5755–5760
Zhu M K, Xu X Y, Gong L Z. Organocatalytic asymmetric syn-aldol reactions of aldehydes with long-chain aliphatic ketones on water and with dihydroxyacetone in organic solvents. Adv Synth Catal, 2008, 350: 1390–1396
Frisch M J, Trucks G W, Schlegel H B, et al. Gaussian 03, Revision C.01, Gaussian: Wallingford, CT, 2004
Becke A D. A new mixing of hartree-fock and local density-functional theories. J Chem Phys, 1993, 98: 1372–1377
Becke A D. Density-functional thermochemistry 3. The role of exact exchange. J Chem Phys, 1993, 98: 5648–5652
Becke A D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A, 1988, 38: 3098–3100
Lee C, Yang W, Parr R G. Development of the colle-salvetti correlation- energy formula into a functional of the electron density. Phys Rev B, 1988, 37: 785–789
Fu A P, Li H L, Tian F H, et al. Theoretical studies of stereoselectivities in the direct anti- and syn-aldol reactions catalyzed by different amino acid derivatives. Tetrahedron: Asymmetry, 2008, 19: 1288–1296
Chen Y, Ye S, Jiao L, et al. Mechanistic twist of the [8+2] cycloadditions of dienylisobenzofurans and dimethyl acetylenedicar- boxylate: Stepwise [8+2] versus [4+2]/[1,5]-vinyl shift mechanisms revealed through a theoretical and experimental study. J Am Chem Soc, 2007, 129: 10773–10784
Shen Y R, Ostroverkhov V. Sum-frequency vibrational spectroscopy on water interfaces: Polar orientation of water molecules at interfaces. Chem Rev, 2006, 106: 1140–1154
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Rao, Q., Luo, S. & Gong, L. Water as dual functional cocatalyst: A theoretical study on the mechanism of direct aldol reaction on water catalyzed by a leucine derivative. Chin. Sci. Bull. 55, 1742–1752 (2010). https://doi.org/10.1007/s11434-010-3059-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11434-010-3059-x