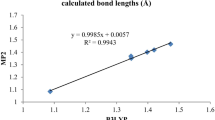
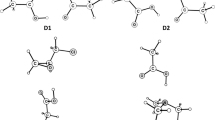
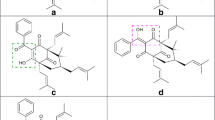
Abstract
The role of intramolecular hydrogen bonds and the presence of electron withdrawing groups in the acidity of secondary aldimines and secondary ketimines is investigated by means of density functional theory simulations. We have found that the presence of an intramolecular hydrogen bond can increase the acidity up to ~ 20 kJ mol−1 with respect to structural isomers not showing it. In general, the excess of negative charge in the deprotonated species is hosted by the electron withdrawing group, thus stabilizing the anion and increasing the acidity. Among the studied structures, secondary ketimines, bearing a phenyl group, have shown to present the highest acidity and are therefore potential candidates that would be used for different Michael and nucleophilic additions in the synthesis of important pharmaceutical and natural products.












Similar content being viewed by others
References
Jeffrey GA (1997) An introduction to hydrogen bonding. Oxford University Press, Oxford
Steiner T (2002) The hydrogen bond in the solid state. Angew Chem Int Ed 41(1):48–76. https://doi.org/10.1002/1521-3773(20020104)41:1%3c48:AID-ANIE48%3e3.0.CO;2-U
Aleman J, Parra A, Jiang H, Jorgensen KA (2011) Squaramides: bridging from molecular recognition to bifunctional organocatalysis. Chem Eur J 17(25):6890–6899. https://doi.org/10.1002/chem.201003694
Zhang Z, Schreiner PR (2009) (Thio)urea organocatalysis-what can be learnt from anion recognition? Chem Soc Rev 38:1187–1198. https://doi.org/10.1039/B801793J
Huang Y, Unni AK, Thadani AN, Rawal VH (2003) Single enantiomers from a chiral-alcohol catalyst. Nature 424:146. https://doi.org/10.1038/424146a
Badiola E, Fiser B, Gómez-Bengoa E, Mielgo A, Olaizola I, Urruzuno I, García JM, Odriozola JM, Razkin J, Oirabide M, Palomo C (2014) Enantioselective construction of tetrasubstituted stereogenic carbons through Brønsted base catalyzed Michael reactions: α′-hydroxy enones as key enoate equivalent. J Am Chem Soc 136(51):17869–177881. https://doi.org/10.1021/ja510603w
Talavera G, Reyes E, Vicario JL, Carrillo L (2012) Cooperative dienamine/hydrogen-bonding catalysis: enantioselective formal [2 + 2] cycloaddition of enals with nitroalkenes. Angew Chem Int Ed 51(17):4104–4107. https://doi.org/10.1002/anie.201200269
Jung CK, Krische MJ (2006) Asymmetric Induction in hydrogen-mediated reductive aldol additions to α-amino aldehydes catalyzed by rhodium: selective formation of syn-stereotriads directed by intramolecular hydrogen-bonding. J Am Chem Soc 128(51):17051–17056. https://doi.org/10.1021/ja066198q
Inokuma T, Hoashi Y, Takemoto Y (2006) Thiourea-catalyzed asymmetric michael addition of activated methylene compounds to α, β-unsaturated Imides: dual activation of imide by intra- and intermolecular hydrogen bonding. J Am Chem Soc 128(29):9413–9419. https://doi.org/10.1021/ja061364f
Wang P, Li HF, Zhao JZ, Du ZH, Da CS (2017) Organocatalytic enantioselective cross-aldol reaction of o-hydroxyarylketones and trifluoromethyl ketones. Org Lett 19(10):2634–2637. https://doi.org/10.1021/acs.orglett.7b00828
Esteban F, Cieslik W, Arpa EM, Guerrero-Corella A, Díaz-Tendero S, Perles J, Fernández-Salas JA, Fraile A, Alemán J (2018) Intramolecular hydrogen bond activation: thiourea- organocatalyzed enantioselective 1,3-dipolar cycloaddition of salicylaldehyde-derived azomethine ylides with nitroalkenes. ACS Catal 8(3):1884–1890. https://doi.org/10.1021/acscatal.7b03553
Guerrero-Corella A, Esteban F, Iniesta M, Martín-Somer A, Parra M, Díaz-Tendero S, Fraile A, Alemán J (2018) 2-Hydroxybenzophenone as chemical auxiliary for the activation of ketiminoesters in the highly enantioselective addition to nitroalkenes under bifunctional catalysis. Angew Chem Int Ed 57(19):5350–5354. https://doi.org/10.1002/anie.201800435
Choubane H, Garrido-Castro AF, Alvarado C, Martín-Somer A, Guerrero-Corella A, Daaou M, Díaz-Tendero S, Maestro MC, Fraile A, Alemán A (2018) Intramolecular hydrogen-bond activation for the addition of nucleophilic imines: 2-hydroxybenzophenone as a chemical auxiliary. Chem Commun 54:3399–3402. https://doi.org/10.1039/C8CC01479E
Arpa EM, Frías M, Alvarado C, Alemán J, Díaz-Tendero S (2016) Weakly bonded intermediates as a previous step towards highly enantioselectivity iminium type additions of beta-keto-sulfoxides and sulfones. J Mol Catal A Chem 423:308–318. https://doi.org/10.1016/j.molcata.2016.03.013
Martín-Sómer A, Arpa EM, Díaz-Tendero S, Alemán J (2018) A density functional theory study of intramolecular hydrogen bond activation of aza-methylene imines in hydrogen bond bifunctional catalysis. Eur J Org Chem. https://doi.org/10.1002/ejoc.201801208
Frisch et al (2013) Gaussian 09, revision E.01. Gaussian, Inc., Wallingford CT
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Acc 120(1–3):215–241. https://doi.org/10.1007/s00214-007-0310-x
Hohenstein EG, Chill ST, Sherrill D (2008) Assessment of the performance of the M05−2X and M06−2X exchange-correlation functionals for noncovalent interactions in biomolecules. J Chem Theory Comput 4(12):1996–2000. https://doi.org/10.1021/ct800308k
Thanthirwatte KS, Hohenstein EG, Burns LA, Sherrill CD (2011) Assessment of the performance of DFT and DFT-D methods for describing distance dependence of hydrogen-bonded interactions. J Chem Theory Comput 7(1):88–96. https://doi.org/10.1021/ct100469b
Walker M, Harvey AJA, Sen A, Dessent CEH (2013) Performance of M06, M06-2X, and M06-HF density functionals for conformationally flexible anionic clusters: M06 functionals perform better than B3LYP for a model system with dispersion and ionic hydrogen-bonding interactions. J Phys Chem A 117(47):12590–12600. https://doi.org/10.1021/jp408166m
Foster JP, Weinhold F (1980) Natural hybrid orbitals. J Am Chem Soc 102(24):7211–7218. https://doi.org/10.1021/ja00544a007
NBO Version 3.1, Glendening ED, Reed AE, Carpenter JE, Weinhold F
Bader RFW (1990) Atoms in molecules: a quantum theory. Clarendon Press, Oxford
Bader RFW (1991) A quantum theory of molecular structure and its applications. Chem Rev 91(5):893–928. https://doi.org/10.1021/cr00005a013
AIMAll (Version 17.11.14), Todd A. Keith, TK Gristmill Software, Overland Park KS, USA, 2017. aim.tkgristmill.com
Fifen JJ, Dhaouadi Z, Nsangou M (2014) Revision of the thermodynamics of the proton in gas phase. J Phys Chem A 118(46):11090–11097. https://doi.org/10.1021/jp508968z
Marenich AV, Cramer CJ, Truhlar DG (2009) Universal solvation model based on solute electron density and a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J Phys Chem B 113:6378–6396. https://doi.org/10.1021/jp810292n
Tosovic J, Markovic S, Milenkovic D, Markovic Z (2016) Solvation enthalpies and Gibbs energies of the proton and electron—influence of solvation models. J Serb Soc Comput Mech 10:66–76. https://doi.org/10.5937/jsscm1602066T
Markovic Z, Tosovic J, Milenkovic D, Markovic S (2016) Revisiting the solvation enthalpies and free energies of the proton and electron in various solvents. Comput Theor Chem 1077:11–17. https://doi.org/10.1016/j.comptc.2015.09.007
Aguilar-Galindo F, Ocón P, Poyato JML (2017) Exploring the catalytic efficiency of X-doped (X = B, N, P) graphene in oxygen reduction reaction: influence of solvent and border effects. Int J Quantum Chem 118:e25579. https://doi.org/10.1002/qua.25579
Acknowledgements
The authors acknowledge the generous allocation of computer time at the Centro de Computación Científica of the Universidad Autonónoma de Madrid (CCC-UAM). This work was partially supported by the projects CTQ2016-76061-P & CTQ2015-64561-R of the Spanish Ministerio de Economía y Competitividad (MINECO). F.A.G. acknowledges the FPI grant associated with the project CTQ2013-43698-P (MINECO). Financial support from the MINECO through the “María de Maeztu” Program for Units of Excellence in R&D (MDM-2014-0377) is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Published as part of the special collection of articles derived from the 11th Congress on Electronic Structure: Principles and Applications (ESPA-2018).
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Aguilar-Galindo, F., Tuñón, A.M., Fraile, A. et al. Role of intramolecular hydrogen bonds and electron withdrawing groups in the acidity of aldimines and ketimines: a density functional theory study. Theor Chem Acc 138, 59 (2019). https://doi.org/10.1007/s00214-019-2451-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-019-2451-0