Abstract
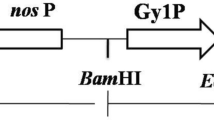
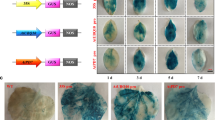
Glucagon-like peptide-1 (GLP-1) is a 30-amino acid peptide hormone, which has the regulatory function of stimulating the secretion of insulin to balance blood glucose levels. In this study, marker-free expression vector pX6 with a fusion gene of ten tandem repeated GLP-1 analog ([Ser8, Gln26 and Asp34]-GLP-1) genes was constructed and transformed into cucumbers by Agrobacterium tumefaciens-mediated transformation method. Four transgenic lines were obtained by PCR and Southern blotting analysis and two transgenic lines successfully expressed the fusion protein (named GLP-T), which was revealed by Western blotting analysis. The biological activity test results showed that the serum glucose level of diabetic rats was significantly decreased after oral administration of the fusion protein GLP-T extracted from the transgenic cucumber fruitage. These results suggest that this may provide a brand-new strategy to prevent and cure the diabetes with no pain.
Similar content being viewed by others
References
Orskov C, Holst J J Nielsen O V. Effect of truncated glucagon-like peptide-1 [proglucagon-(78-107) amide] on endocrine secretion from pig pancreas, antrum, and nonantral stomach. Endocrinology, 1988, 123: 2009–2013
Wettergren A, Schjoldager B, Mortensen P E, et al. Truncated GLP-1 (proglucagon78-107-amide) inhibits gastric and pancreatic functions in man. Dig Dis Sci, 1993, 38: 665–673
Layer P, Holst J J Grandt D, et al. Ileal release of glucagonlikepeptide-1 (GLP-1). Association with inhibition of gastric acid secretion in humans. Dig Dis Sci, 1995, 40: 1074–1082
Hou J H Yan R X Yang L Q, et al. High-level expression of fusion protein containing 10 tandem repeated GLP-1 analogs in yeast Pichia pastoris and its biological activity in a diabetic rat model. Biosci Biotechnol Biochem, 2007, 71: 1462–1469
Mentlein R, Gallwitz B, Schimdt W E. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagons-like peptide-1(7-36) amide, peptide histidine methionine, and is responsible for their degradation in human serum. Eur J Biochem, 1993, 214: 829–835
Vilsboll T, Agerso H, Krarup T, et al. Similar elimination rates of glucagon-like peptide-1 in obese type 2 diabetic patients and healthy subjects. J Clin Endocrinol Metab, 2003, 88: 220–224
Erickson L, Yu W J Zhang J, et al. The Production and Delivery of Therapeutic Peptides in Plants. In: Erickson L, Yu W J, Brandle J et al., eds. Molecular Farming of Plants and Animals for Human and Veterinary Medicine. Dordrecht, Netherlands: Kluwer Academic Publishers, 2002. 197–222
Larrick J W Yu L, Naftzger C, et al. Human Pharmaceuticals Produced in Plants. In: Hood E E, Howard J A, eds. Plants as Factories for protein production. Dordrecht, Netherlands: Kluwer Academic Publishers, 2002. 79-101
Hou J H, Expression of oral long-acting GLP-1 in yeast Pichia pastoris and Escherichia coli. Doctor Dissertation. Tianjin: Nankai University, 2007
Wang G L Fang H J. Genetic Engineering of Plant, Beijing: Science Press, 2002, 770–777
Paula P. Transformation of Cucumis Sativus tissue by Agrobacterium tumefaciens and the regeneration of transformed plants. Plant Cell Report, 1990, 9: 245–248
Murray M G Thompson W F. Rapid isolation of high molecular weight plant DNA. Nucleic Acid Res, 1980, 8: 4321–4325
Tada Y, Utsumi S, Akaiwa F. Foreign gene products can be enhanced by introduction into low storage protein mutants. Plant Biotechnol J, 2003, 1: 411–422
Burcelin R, Eddouks M, Maury J, et al. Excessive glucose production, rather than insulin resistance, accounts for hyperglycaemia in recent-onset streptozotocin-diabetic rats. Diabetologia, 1995, 38: 283–290
Zuo J R Niu Q, Moller S, et al. Chemical-regulated, site-specific DNA recombination in transgenic plants. Nat Biotechnol, 2001, 19: 157–161
Ishige F, Takaichi M, Foster R, et al. A G-box motif (GCCACGTG CC) tetramer confers high-level constitutive expression in dicot and monocot plants. Plant J, 1999, 18: 443–448
Giddings G, Allison G, Brooks D, et al. Transgenic plants as factories for biopharmaccuticals. Nat Biotech, 2000, 18: 1151–1155
Daniell H, Streatfield S, Wycoff K. Medical molecular farming: production of antibodies, biopharmaceuticals and edible vaccines in plants. Trend Plant Sci, 2001, 6: 219–226
Giddings G. Transgenic plants as protein factories. Curr Opin Biotechnol, 2001, 12: 450–454
Koichi S, Saori E K Yoshifumi T. Genetically modified rice seeds accumulating GLP-1 analogue stimulate insulin secretion from a mouse pancreatic beta-cell line. FEBS Lett, 2005, 579: 1085–1088
Hiroshi Y, Yoshifumi T, Yuji H, et al. Expression of the small peptide GLP-1 in transgenic plants. Transgenic Research, 2005, 14: 677–684
Francois I E J A, de Bolle M F C Dwyer G, et al. Transgenic expression in Arabidopsis of a polyprotein construct leading to production of two different antimicrobial proteins. Plant Physiol, 2002, 128: 1346–1358
Spiker S, Thompson W F. Nuclear matrix attachment regions and transgene expression in plants. Plant Physiol, 1996, 110: 15–21
Gallie D R. Controlling gene expression in transgenics. Curr Opin Plant Biol, 1998, 1: 166–172
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the Technology Key Project of Tianjin Technology Committee Funds of China (Grant Nos. 06YFGZNC00700, 08ZCKFSH00100) and National Hi-Tech Research and Development Program of China (Grant Nos. 2007AA06Z323, 2008AA02Z205)
About this article
Cite this article
Zhao, L., Liao, F., Wang, C. et al. Generation of transgenic cucumbers with expression of a ten-tandem repeat long-acting GLP-1 analogue and their biological function on diabetic rats. Chin. Sci. Bull. 54, 4658–4663 (2009). https://doi.org/10.1007/s11434-009-0699-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11434-009-0699-9