Abstract
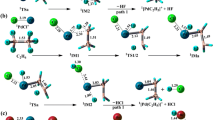
The insertion reaction mechanism of CBr2 with CH3CH2O has been studied by using the B3LYP/6-311G(d) and CCSD(T)/6-311G(d) at single point. The geometries of reactions, transition state and products were completely optimized. All the transition state is verified by the vibrational analysis and the internal reaction coordinate (IRC) calculations. The results show that reaction (1) is the dominant reaction path, which proceeds via two steps: i) two reactants form an intermediate (IM1), which is an exothermal reaction of 8.62 kJ·mol−1 without energy barrier; ii) P1 is obtained via the TS1 and the H-shift, in which the energy barrier is 44.53 kJ·mol−1. The statistical thermodynamics and Eyring transition state theory with Wigner correction are used to study the thermodynamic and kinetic characters of this reaction in temperature range from 100 to 2200 K. The results show that the appropriate reaction temperature ranges from 200 to 1900 K at 1.0 atm, in which the reaction has a bigger spontaneity capability, equilibrium constant (K) and higher rate constant (k).
Similar content being viewed by others
References
Houk K N, Rondan N G, Cielo S, et al. Theoretical studies of the structures and reactions of substituted carbonyl ylides. J Am Chem Soc, 1980, 102(5): 1504–1512
Gill H S, Landgrebe J A. Selective formation and trapping of dihalocarbonyl ylides derived from dihalocarbenes and substituted benzaldenhydes. J Org Chem, 1983, 48(7): 1051–1055
Hu Y M, Hira K, Tomioka H. Generation and reaction of triplet bis(2,6-dimethyl-4-methoxyphenyl)carbene. Acta Chim Sin (in Chinese), 2002, 60(11): 2056–2063
Seyferth D, Burlitch J M, Minasz R J, et al. Halomethyl-metal compounds. II. The preparation of gem-dihalocyclopropanes by the reaction of phenyl(trihalomethyl)mercury compounds with olefins. J Am Chem Soc, 1965, 87(19): 4259–4270
Seyferth D, Tronich W. Reactions of phenyl (bromodichloromethyl) mercury. Preparation of perchlorothiirane. J Am Chem Soc, 1969, 91(8): 2138–2139
Seyferth D, Darragh K V. Halomethyl-metal compounds. XXXI. Phenyl(fluorodichloromethyl)mercury. A useful source of fluorochlorocarbene. J Org Chem, 1970, 35(5): 1297–1302
Houk K N, Rondan N G. Theoretical studies of halocarbene cycloaddition selectivities: A new interpretation of negative activation energies and entropy control of selectivity. Tetrahedron, 1985, 41(8): 1555–1563
Keating A E, Mrrigan S R, Singleton D A, et al. Experimental proof of the non-least-motion cycloadditions of dichlorocarbene to alkenes: kinetic isotope effects and quantum mechanical transition states. J Am Chem Soc, 1999, 121(16): 3933–3938
Wang H X, Huan Z W, Zhang G J, et al. Recent development in understanding the reactions of dihalocarbenes with carbonyl compounds. Chem J Chin Univ (in Chinese), 1996, 17(2): 241–248
Gracia L, Sambrano J R, Safont V S, et al. Theoretical study on the molecular mechanism for reaction of VO2 + with C2H4. J Phys Chem A, 2003, 107(17): 3107–3120
Li M, Zheng W X, Tian A M. Density functional study on enantioselective reduction of keto oxime ether with borane catalyzed by oxazaborolidine. Sci China Ser B-Chem, 2006, 49(4): 296–307
Lu X H, Yu H B, Xü Y H, et al. Theoretical study on the mechanism of the cycloaddition reaction between methylidenesilene and ethylene. Chin J Chem, 2006, 24(3): 307–310
Lu X H, Wu W R, Yu H B, et al. Theoretical study on the mechanism of cycloaddition between dimethyl methylene carbene and acetone. Chin Sci Bull, 2005, 50(20): 2281–2287
Peng L, Li Q S, Fang W H, et al. Theoretical study on cycloaddition of singlet dichlorocarbene with formaldehyde, acetaldehyde and benzaldehyde and subsequent rearrangement reactions. Chem Phys Lett, 2003, 382(1): 126–132
Takashi H, Tomoki K, Tomomasa K, et al. Carbene insertion into oxygen-hydrogen bonds by metalloporphyrin catalysts. J Organomet Chem, 1994, 473(1): 323–327
Ramalingam M, Ramasami K, Venuvanalingam P, et al. C-H functionalisation through carbene and fluorocarbene insertion—ab initio and DFT investigations. J Mol Struc-Theochem, 2005, 755(1): 169–178
Lin Q J, Feng D C, Ma W Y, et al. Theoretical studies on the C-H bond insertion reaction of carbenes with ether(II)—insertion reactions of CX2(X = F, Cl) with dimethylether. Chem J Chin Univ (in Chinese), 2000, 21(9): 1427–1431
Feng D C, Lin Q J, Ma W Y, et al. Theoretical studies on the C-H bond insertion reaction of carbenes with ether(III)—insertion reactions of CX2(X = H, F, Cl) with ethylmethylether. Chem J Chin Univ (in Chinese), 2000, 21(11): 1708–1712
Lin Q J, Feng D C, Qi C S. Theoretical studies on the C-H bond insertion reaction of carbenes with ether(III)—insertion reactions of CX2(X = H, F, Cl) with benzylmethylether. Chem J Chin Univ (in Chinese), 2000, 21(12): 1922–1924
Chen X Y, Zhao C X, Ping Y, et al. Theoretical study of reactions between MH (M=B, Al) and the H2S molecule. Int J Quant Chem, 2001, 85(3): 127–135
Si W J, Ju G Z. Thermodynamic and kinetic studies on the reaction of silylidyne insertion into HF. Chin J Inorg Chem (in Chinese), 2002, 18(8): 782–786
Si W J, Zhuo S P, Ju G Z. Thermodynamic and kinetic investigations on NH+O3→ONH+O2 reaction. Acta Phys-Chim Sin (in Chinese), 2003, 19(10): 974–977
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the fund of Tianshui Normal University (Grant No. TSA0604)
About this article
Cite this article
Li, Z., Lü, L., Kang, J. et al. Density functional theory study on the insertion reaction mechanism of dibromocarbene with formaldehyde. CHINESE SCI BULL 52, 2035–2041 (2007). https://doi.org/10.1007/s11434-007-0289-7
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11434-007-0289-7