Abstract
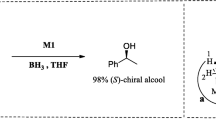
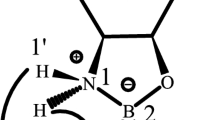
The enantioselective reduction of keto oxime ether with borane catalyzed by oxazaborolidine is discussed by the density functional theory (DFT) method. The main intermediates and transition states for this reaction are optimized completely at the B3LYP/6-31g(d) level, and the transition states are verified by vibrational modes. As shown, the chirality-controlled steps for this reaction are the hydride transfer from borane to carbonyl carbon and oxime carbon of keto oxime ether, and the chirality for the reduced products is determined in these two reaction steps. In all examined reaction paths, the first hydride is transferred via a six-membered ring and the second hydride via a five-membered ring or a four-membered ring.
Similar content being viewed by others
References
Noyori R, Kitamura M. Enantioselective addition of organometallic reagents to carbonyl compounds: Transfer, duplication and intestification of chirality. Angew Chem, Int Ed Engl, 1991, 30: 34–48
Soai K, Niwa S. Enantioselective addition of organozine reagents to aldehydes. Chem Rev, 1992, 92: 833–856
Blaser H U. The chiral pool as a source of enantioselective catalysts and auxiliaries. Chem Rev, 1992, 92: 935–952
Tillyer R D, Baudreau C, Tschaen D, Dolling U H, Reider P J. Asymmetric reduction of keto oxime ethers using oxazaborolidine reagents: The enantioselective synthesis of cyclic amino alcohols. Tetrahedron Lett, 1995, 36: 4337–4340
Nevalainen V. Quantum chemical modeling of chiral catalysis: Part 3. On the role of a Lewis basic solvent in the mechanism of catalytic enantioselective reduction of carbonyl compounds by chiral oxazaborolidines. Tetrahedron: Asymmetry, 1991, 2: 827–842
Nevalainen V. Quantum chemical modeling of chiral catalysis: Part 4. On the hydride transfer in ketone complexes of borane adducts of oxazaborolidines and regeneration of catalyst. Tetrahedron: Asymmetry, 1991, 2: 1133–1155
Nevalainen V. Quantum chemical modeling of chiral catalysis: Part 8. On the conformational freedom of the ketone of ketone-borane complexes of oxazaborolidines used as catalysts in the enantioselective reduction of ketones. Tetrahedron: Asymmetry, 1992, 3: 1563–1572
Nevalainen V. Quantum chemical modeling of chiral catalysis: Part 7. On the effects controlling the coordination of borane to chiral oxazaborolidines used as catalysts in the enantioselective reduction of ketones. Tetrahedron: Asymmetry, 1992, 3: 1441–1453
Nevalainen V. Quantum chemical modeling of chiral catalysis: Part 5. On the role of alkoxyboranes in the catalytic enantioselective reduction of carbonyl compounds by the Corey-Bakshi-Shibata method. Tetrahedron: Asymmetry, 1992, 3: 921–932
Nevalainen V. Quantum chemical modeling of chiral catalysis: Part 12. On the influence of the nature of the ring system on binding in ketone-borane complexes of chiral oxazaborolidines used as catalysts in the enantioselective reduction of ketones. Tetrahedron: Asymmetry, 1993, 4: 1597–1602
Nevalainen V. Quantum chemical modeling of chiral catalysis: Part 19. Strain and stability-oxazadiboretanes potentially involved in the enantioselective reduction of ketones promoted by chiral oxazaborolidines. Tetrahedron: Asymmetry, 1994, 5: 903–908
Linney L P, Self C R, Williams I H. A theoretical investigation of hydride bridging in chiral oxazaborolidine-borane adducts: The importance of electron correlation. Tetrahedron: Asymmetry, 1994, 5: 813–816
Quallich G J, Blake J F, Woodall T M. A combined synthetic and ab initio study of chiral oxazaborolidines structure and enantioselectivity relationships. J Am Chem Soc, 1994, 116: 8516–8525
Nevalainen V, Uggla R, Sundberg M R. A new class of oxazaborolidine derivatives discovered, ketones and aldehydes as bidentate ligands. Tetrahedron: Asymmetry, 1995, 6: 1431–1440
Nevalainen V. HB(OH)2 and H2CO as probes for a study on binding of dialkoxyboranes and ketones to oxazaborolidines capable of catalyzing the enantioselective reduction of ketones. Tetrahedron: Asymmetry, 1996, 7: 2655–2664
Uggla R, Nevalainen V, Sundberg M R. On the role of π-stacking in aldehyde complexes of N-solfonylated oxazaborolidines used as chiral catalysts. Tetrahedron: Asymmetry, 1996, 7: 2725–2732
Nevalainen V. Borane as a model of Lewis acidic activators of chiral N-solfonylated oxazaborolidines used to catalyze asymmetric Diels-Alder reactions. Tetrahedron: Asymmetry, 1996, 7: 1449–1456
Nevalamen V. A computational study on the activation of chiral N-solfonylated oxazaborolidines by Lewis acids. Tetrahedron: Asymmetry, 1997, 8: 2241–2248
Corey E J, Bakshi R K, Shibata S. Highly enantioselective borane reduction ketones catalyzed by chiral oxazaborolidines. J Am Chem Soc, 1987, 109: 5551–5553
Li M, Zheng W X, Yang F, Tian A M. Quantum chemical study on enantioselective reduction of keto oxime ether with borane catalyzed by oxazaborolidine: Part 1. Structures of catalyst-boraneketo oxime ether adducts. Intern J Quant Chem, 2001, 81: 291–306
Li M, Zheng W X, He R X, Tian A M. Quantum chemical study on enantioselective reduction of keto oxime ether with borane catalyzed by oxazaborolidine: Part 2. The reduction process through the formation of catalyst-alkoxyborane adducts. Intern J Quant Chem, 2003, 93: 294–306
Li M, Zheng W X, Tian A M. Quantum chemical study on enantioselective reduction of keto oxime ether with borane catalyzed by oxazaborolidine: Part 3. The reduction process through the formation of a 7-membered ring adducts. Intern J Quant Chem, 2003, 93: 307–316
Zheng W X, Li M, Tian A M. Quantum chemical study on enantioselective reduction of keto oxime ether with borane catalyzed by oxazaborolidine: Part 4. The reduction process in which oxime is reduced first and carbonyl is reduced through an intermediate containing a B(2)-N(3)-BBH2-OC=O-CC=O-CC=N-NC=N 7-membered ring. J Mol Struct (Theochem), 2003, 634: 253–264
Frisch M J, Trucks G W, Schlegel H B, Scuseria G E, Robb M A, Cheeseman J R, Montgomery J A, Vreven Jr T, Kudin K N, Burant J C, Millam J M, Iyengar S S, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson G A, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox J E, Hratchian H P, Cross J B, Adamo C, Jaramillo J, Gomperts R, Stratmann R E, Yazyev O, Austin A J, Cammi R, Pomelli C, Ochterski J W, Ayala P Y, Morokuma K, Voth G A, Salvador P, Dannenberg J J, Zakrzewski V G, Dapprich S, Daniels A D, Strain M C, Farkas O, Malick D K, Rabuck A D, Raghavachari K, Foresman J B, Ortiz J V, Cui Q, Baboul A G, Clifford S, Cioslowski J, Stefanov B B, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin R L, Fox D J, Keith T, Al-Laham M A, Peng C Y, Nanayakkara A, Challacombe M, Gill P M W, Johnson B, Chen W, Wong M W, Gonzalez C, Pople J A. Gaussian 03, Revision B.03. Pittsburgh: Gaussian, Inc, 2003
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, M., Zheng, W. & Tian, A. Density functional study on enantioselective reduction of keto oxime ether with borane catalyzed by oxazaborolidine. SCI CHINA SER B 49, 296–307 (2006). https://doi.org/10.1007/s11426-006-2008-7
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11426-006-2008-7