Abstract
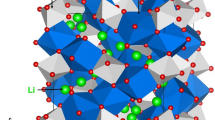
As an ion conductor, the Al-doped Li1+xAlxGe2−x(PO4)3 (LAGP) demonstrates the superionic Li diffusion behavior, however, without the convinced verifications. In this context, the density functional theory (DFT) calculations are employed to clarify the structural origin of the fast Li ion migration kinetics in LAGP solid electrolytes. The calculated results show that doping of Al leads to an emerging high-energy 36f Li site, which plays an important role in promoting the Li diffusion and can largely lower the Li ion diffusion energy barrier. Moreover, the Li/Al antisite defect is investigated firstly, with which the Li ions are excited to occupy a relatively high energy site in LAGP. The obvious local structural distortion by Li/Al antisite results in the coordination change upon Li diffusion (lattice field distortion), which facilitates the Li diffusion significantly and is probably the main reason to account for the superionic diffusion phenomenon. Therefore, the occupation of Li at high-energy sites should be an effective method to establish the fast Li diffusion, which implies a rewarding avenue to build better Li-ion batteries.
Similar content being viewed by others
References
Zubi G, Dufo-López R, Carvalho M, et al. The lithium-ion battery: State of the art and future perspectives. Renew Sustain Energy Rev, 2018, 1: 292–308
Liu K, Liu Y, Lin D, et al. Materials for lithium-ion battery safety. Sci Adv, 2018, 4: eaas9820
Wu X, Song K, Zhang X, et al. Safety issues in lithium ion batteries: Materials and cell design. Front Energy Res, 2019, 7: 65
Francisco B E, Stoldt C R, M’Peko J C. Energetics of ion transport in NASICON-type electrolytes. J Phys Chem C, 2015, 1: 16432–16442
Weiss M, Weber D A, Senyshyn A, et al. Correlating transport and structural properties in Li1+xAlxGe2−x(PO4)3 (LAGP) prepared from aqueous solution. ACS Appl Mater Interfaces, 2018, 1: 10935–10944
Meesala Y, Chen C Y, Jena A, et al. All-solid-state Li-ion battery using Li1.5Al0.5Ge1.5(PO4)3 as electrolyte without polymer interfacial adhesion. J Phys Chem C, 2018, 1: 14383–14389
Sun Z, Liu L, Lu Y, et al. Preparation and ionic conduction of Li1.5-Al0.5Ge1.5(PO4)3 solid electrolyte using inorganic germanium as precursor. J Eur Ceramic Soc, 2019, 1: 402–408
Zhao E, Ma F, Jin Y, et al. Pechini synthesis of high ionic conductivity Li1.3Al0.3Ti1.7(PO4)3 solid electrolytes: The effect of dispersant. J Alloys Compd, 2016, 1: 646–653
Pérez-Estébanez M, Isasi-Marín J, Többens D M, et al. A systematic study of NASICON-type Li1+xMxTi2−x(PO4)3 (M: Cr, Al, Fe) by neutron diffraction and impedance spectroscopy. Solid State Ion, 2014, 1: 1–8
Huang L, Wen Z, Wu M, et al. Electrochemical properties of Li1.4-Al0.4Ti1.6(PO4)3 synthesized by a co-precipitation method. J Power Sources, 2011, 1: 6943–6946
Zhang M, Huang Z, Cheng J, et al. Solid state lithium ionic conducting thin film Li1.4Al0.4Ge1.6(PO4)3 prepared by tape casting. J Alloys Compd, 2014, 1: 147–152
Waetzig K, Rost A, Heubner C, et al. Synthesis and sintering of Li1.3Al0.3Ti1.7(PO4)3 (LATP) electrolyte for ceramics with improved Li+ conductivity. J Alloys Compd, 2020, 818: 153237
Feng J K, Lu L, Lai M O. Lithium storage capability of lithium ion conductor Li1.5Al0.5Ge1.5(PO4)3. J Alloys Compd, 2010, 1: 255–258
He K, Zu C, Wang Y, et al. Stability of lithium ion conductor NASICON structure glass ceramic in acid and alkaline aqueous solution. Solid State Ion, 2014, 1: 78–81
Hull S. Superionics: Crystal structures and conduction processes. Rep Prog Phys, 2004, 1: 1233–1314
Wan T H, Lu Z, Ciucci F. A first principle study of the phase stability, ion transport and substitution strategy for highly ionic conductive sodium antipervoskite as solid electrolyte for sodium ion batteries. J Power Sources, 2018, 1: 61–70
Lang B, Ziebarth B, Elsässer C. Lithium ion conduction in LiTi2(PO4)3 and related compounds based on the NASICON structure: A first-principles study. Chem Mater, 2015, 1: 5040–5048
Rossbach A, Tietz F, Grieshammer S. Structural and transport properties of lithium-conducting NASICON materials. J Power Sources, 2018, 1: 1–9
He X, Zhu Y, Mo Y. Origin of fast ion diffusion in super-ionic conductors. Nat Commun, 2017, 8: 15893
Li H, Okamoto N L, Hatakeyama T, et al. Fast diffusion of multivalent ions facilitated by concerted interactions in dual-ion battery systems. Adv Energy Mater, 2018, 8: 1801475
Gao A, Li M, Guo N, et al. K-birnessite electrode obtained by ion exchange for potassium-ion batteries: Insight into the concerted ionic diffusion and K storage mechanism. Adv Energy Mater, 2019, 9: 1802739
Zhang Z, Zou Z, Kaup K, et al. Correlated migration invokes jigher Na+-ion conductivity in NaSICON-type solid electrolytes. Adv Energy Mater, 2019, 9: 1902373
Siqi S, Jian G, Yue L, et al. Multi-scale computation methods: Their applications in lithium-ion battery research and development. Chin Phys B, 2016, 25: 018212
Liu Q, Li S, Wang S, et al. Kinetically determined phase transition from stage II (LiC12) to stage I (LiC6) in a graphite anode for Li-ion batteries. J Phys Chem Lett, 2018, 1: 5567–5573
Sun Y, Lu X, Xiao R, et al. Kinetically controlled lithium-staging in delithiated LiFePO4 driven by the Fe center mediated interlayer Li-Li interactions. Chem Mater, 2012, 1: 4693–4703
Gao Y, Ma J, Wang X, et al. Improved electron/Li-ion transport and oxygen stability of Mo-doped Li2MnO3. J Mater Chem A, 2014, 1: 4811–4818
Sun Y, Zhao L, Pan H, et al. Direct atomic-scale confirmation of three-phase storage mechanism in Li4Ti5O12 anodes for room-temperature sodium-ion batteries. Nat Commun, 2013, 4: 1870
He Y, Sun Y, Lu X, et al. First-principles prediction of fast migration channels of potassium ions in KAlSi3O8 hollandite: Implications for high conductivity anomalies in subduction zones. Geophys Res Lett, 2016, 1: 6228–6233
Huang Y, He Y, Sheng H, et al. Li-ion battery material under high pressure: Amorphization and enhanced conductivity of Li4Ti5O12. Natl Sci Rev, 2019, 1: 239–246
He Y, Lu X, Kim D Y. A first-principles study on Si24 as an anode material for rechargeable batteries. RSC Adv, 2018, 1: 20228–20233
Zhang Q, Zhang S, Ning F, et al. Calcium doping of lithium titanium oxide nanospheres: A combined first-principles and experimental study. Energy Technol, 2017, 1: 539–543
Redhammer G J, Rettenwander D, Pristat S, et al. A single crystal X-ray and powder neutron diffraction study on NASICON-type Li1+x-AlxTi2−x(PO4)3 (0 ≤ x ≤ 0.5) crystals: Implications on ionic conductivity. Solid State Sci, 2016, 1: 99–107
Monchak M, Hupfer T, Senyshyn A, et al. Lithium diffusion pathway in Li1.3Al0.3Ti1.7(PO4)3 (LATP) superionic conductor. Inorg Chem, 2016, 1: 2941–2945
Arbi K, Hoelzel M, Kuhn A, et al. Local structure and lithium mobility in intercalated Li3AlxTi2−x(PO4)3 NASICON type materials: A combined neutron diffraction and NMR study. Phys Chem Chem Phys, 2014, 1: 18397–18405
Okhotnikov K, Charpentier T, Cadars S. Supercell program: A combinatorial structure-generation approach for the local-level modeling of atomic substitutions and partial occupancies in crystals. J Cheminform, 2016, 8: 17
Momma K, Izumi F. VESTA: A three-dimensional visualization system for electronic and structural analysis. J Appl Crystlogr, 2008, 1: 653–658
Kresse G, Hafner J. Ab initio molecular dynamics for liquid metals. Phys Rev B, 1993, 1: 558–561
Kresse G, Furthmüller J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys Rev B, 1996, 1: 11169–11186
Blöchl P E. Projector augmented-wave method. Phys Rev B, 1994, 1: 17953–17979
Perdew J P, Burke K, Ernzerhof M. Generalized gradient approximation made simple. Phys Rev Lett, 1996, 1: 3865–3868
Henkelman G, Uberuaga B P, Jónsson H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J Chem Phys, 2000, 1: 9901–9904
Liu Y, Yuan Y, Liu F, et al. Interaction between magnetic moments and itinerant carriers in d0 ferromagnetic SiC. Phys Rev B, 2017, 95: 195309
Lu X, Wang S, Xiao R, et al. First-principles insight into the structural fundamental of super ionic conducting in NASICON MTi2(PO4)3 (M =Li, Na) materials for rechargeable batteries. Nano Energy, 2017, 1: 626–633
Wang Q, Zhang M, Zhou C, et al. Concerted ion-exchange mechanism for sodium diffusion and its promotion in Na3V2(PO4)3 framework. J Phys Chem C, 2018, 1: 16649–16654
Zhang B, Lin Z, Dong H, et al. Revealing cooperative Li-ion migration in Li1+xAlxTi2−x(PO4)3 solid state electrolytes with high Al doping. J Mater Chem A, 2020, 1: 342–348
Arjmandi H R, Grieshammer S. Defect formation and migration in Nasicon Li1+xAlxTi2−x(PO4)3. Phys Chem Chem Phys, 2019, 1: 24232–24238
Lu Z, Chen C, Baiyee Z M, et al. Defect chemistry and lithium transport in Li3OCl anti-perovskite superionic conductors. Phys Chem Chem Phys, 2015, 1: 32547–32555
Oh K, Chang D, Lee B, et al. Native defects in Li10GeP2S12 and their effect on lithium diffusion. Chem Mater, 2018, 1: 4995–5004
Author information
Authors and Affiliations
Corresponding authors
Additional information
This work was supported by the National Key Research and Development Program of China (Grant No. 2019YFA0705700), National Natural Science Foundation of China (Grant No. 11704019), the Hundreds of Talents Program of Sun Yat-sen University and the Fundamental Research Funds for the Central Universities. Computational resources were provided by the National Supercomputing Center in Shenzhen.
Rights and permissions
About this article
Cite this article
Jiang, C., Lu, X. & Cao, D. First-principles insight into the entanglements between superionic diffusion and Li/Al antisite in Al-doped Li1+xAlxGe2−x(PO4)3 (LAGP). Sci. China Technol. Sci. 63, 1787–1794 (2020). https://doi.org/10.1007/s11431-020-1562-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11431-020-1562-3