Abstract
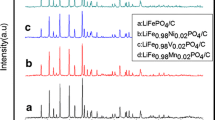
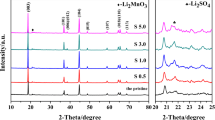
The olivine-type structure LiFe0.65Mn0.35PO4 materials are respectively synthesized via MnO2 and MnC2O4·2H2O as manganese resources by using solid-state reaction. The compound materials are characterized by scanning electron microscopies (SEM), transmission electron microscopy (TEM), X-ray photoelectronspectroscopy (XPS) and electrochemical test. The experimental results demonstrate that LiFe0.65Mn0.35PO4 prepared by MnO2 as manganese resource exhibits uniform particles with porous structure in SEM and TEM images. XPS data show the coexistence of Mn4+, Mn3+ and Mn2+ cations. Besides, this sample shows better discharge special capacity of 107.46 mA h g–1 at 5 C and capacitance conservation rate about 95.47% after 100 cycles at 1 C. The superior electrochemical capability is attributed to the coexistence of mixed-valence manganese cations in crystal and the uniform particles with porous structure.
Similar content being viewed by others
References
Padhi A K. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries. J Electrochem Soc, 1997, 144: 1188–1194
Wang Y, Wang Y, Hosono E, et al. The design of a LiFePO4/Carbon nanocomposite with a core-shell structure and its synthesis by an in situ polymerization restriction method. Angew Chem Int Ed, 2008, 47: 7461–7465
Sun C, Rajasekhara S, Goodenough J B, et al. Monodisperse porous LiFePO4 microspheres for a high power Li-ion battery cathode. J Am Chem Soc, 2011, 133: 2132–2135
Saravanan K, Balaya P, Reddy M V, et al. Morphology controlled synthesis of LiFePO4/C nanoplates for Li-ion batteries. Energy Environ Sci, 2010, 3: 457–464
Nan C, Lu J, Chen C, et al. Solvothermal synthesis of lithium iron phosphate nanoplates. J Mater Chem, 2011, 21: 9994–9996
Ma Z, Shao G, Wang G, et al. Electrochemical performance of Mo-doped LiFePO4/C composites prepared by two-step solid-state reaction. Ionics, 2013, 19: 437–443
Fang H, Liang G, Zhao L, et al. Electrochemical properties of cathode material LiFePO4 with Ti substitution. J Electrochem Soc, 2013, 160: A3148–A3152
Moretti A, Giuli G, Nobili F, et al. Structural and electrochemical characterization of vanadium-doped LiFePO4 cathodes for lithiumion batteries. J Electrochem Soc, 2013, 160: A940–A949
Zhang P, Wang Y, Lin M, et al. Doping effect of Nb5+ on the microstructure and defects of LiFePO4. J Electrochem Soc, 2012, 159: A402
Li C, Hua N, Wang C, et al. Effect of Mn2+-doping in LiFePO4 and the low temperature electrochemical performances. J Alloys Compd, 2011, 509: 1897–1900
Liu Q, Liu Z, Xiao G, et al. Enhancement of capacity at high charge/discharge rate and cyclic stability of LiFePO4/C by nickel doping. Ionics, 2013, 19: 445–450
Čech O, Thomas J E, Visintin A, et al. Cobalt doped LiFePO4/C composite material for Li-ion cathodes. ECS Trans, 2012, 40: 93–98
Wang B, Xu B, Liu T, et al. Mesoporous carbon-coated LiFePO4 nanocrystals co-modified with graphene and Mg2+ doping as superior cathode materials for lithium ion batteries. Nanoscale, 2012, 6: 986–995
Huang X, Liang F, Du Y, et al. Optimization of the process parameters for the synthesis of LiFe1−x−y Mgx Tiy PO4/C cathode material using response surface methodology. Nano, 2016, 11: 1650122
Wang H, Yang Y, Liang Y, et al. LiMn1−x FexPO4 nanorods grown on graphene sheets for ultrahigh-rate-performance lithium ion batteries. Angew Chem Int Ed, 2011, 50: 7364–7368
Yamada A, Chung S C. Crystal chemistry of the olivine-type Li(MnyFe1−y )PO4 and (MnyFe1−y )PO4 as possible 4 V cathode materials for lithium batteries. J Electrochem Soc, 2001, 148: 960–967
Dinh H C, Mho S, Kang Y, et al. Large discharge capacities at high current rates for carbon-coated LiMnPO4 nanocrystalline cathodes. J Power Sources, 2013, 244: 189–195
Delacourt C, Poizot P, Morcrette M, et al. One-step low-temperature route for the preparation of electrochemically active LiMnPO4 powders. Chem Mater, 2004, 16: 93–99
Fisher C A J, Hart Prieto V M, Islam M S. Lithium battery materials LiMPO4 (M=Mn, Fe, Co, and Ni): Insights into defect association, transport mechanisms, and doping behavior. Chem Mater, 2008, 20: 5907–5915
Chen K, Pan W, Xue D. Phase transformation of Ce3+-doped MnO2 for pseudocapacitive electrode materials. J Phys Chem C, 2016, 120: 20077–20081
Chen K, Dong Noh Y, Li K, et al. Microwave-hydrothermal crystallization of polymorphic MnO2 for electrochemical energy storage. J Phys Chem C, 2013, 117: 10770–10779
Zhang Y, Sun C, Lu P, et al. Crystallization design of MnO2 towards better supercapacitance. Cryst Eng Comm, 2014, 14: 5892–5897
Ghodbane O, Pascal J L, Favier F. Microstructural effects on chargestorage properties in MnO2-based electrochemical supercapacitors. ACS Appl Mater Interfaces, 2009, 1: 1130–1139
Wang Y, Lai W, Wang N, et al. A reduced graphene oxide/mixed-valence manganese oxide composite electrode for tailorable and surface mountable supercapacitors with high capacitance and super-long life. Energy Environ Sci, 2017, 10: 941–949
Xu C, Du H, Li B, et al. Capacitive behavior and charge storage mechanism of manganese dioxide in aqueous solution containing bivalent cations. J Electrochem Soc, 2009, 156: A73
Paolella A, Bertoni G, Dilena E, et al. Redox centers evolution in phospho-olivine type (LiFe0.5 Mn0.5PO4 ) nanoplatelets with uniform cation distribution. Nano Lett, 2014, 14: 1477–1483
Dedryvère R, Maccario M, Croguennec L, et al. X-ray photoelectron spectroscopy investigations of carbon-coated LixFePO4 materials. Chem Mater, 2008, 20: 7164–7170
Lv C, Duan X, Deng J, et al. LiFePO4 mesocrystals coated with N-doped carbon from an ionic liquid for Li-ion batteries. Cryst Eng Comm, 2017, 19: 1253–1257
Peng W, Jiao L, Gao H, et al. A novel sol-gel method based on FePO4·2H2O to synthesize submicrometer structured LiFePO4/C cathode material. J Power Sources, 2011, 196: 2841–2847
Hu Y, Zhang T, Cheng F, et al. Recycling application of Li-MnO2 batteries as rechargeable lithium-air batteries. Angew Chem, 2015, 127: 4412–4417
Liu Q L, Wang S P, Cheng H. High rate capabilities Fe-doped EMD electrodes for Li/MnO2 primary battery. Int J Electrochem Sci, 2013, 8: 10540–10548
Cui Y, Zhao X, Guo R. Improved electrochemical performance of La0.7Sr0.3MnO3 and carbon co-coated LiFePO4 synthesized by freeze-drying process. Electrochim Acta, 2010, 55: 922–926
Zhao J, He J, Zhou J, et al. Facile synthesis for LiFePO4 nanospheres in tridimensional porous carbon framework for lithium ion batteries. J Phys Chem C, 2011, 115: 2888–2894
Yu D Y W, Fietzek C, Weydanz W, et al. Study of LiFePO4 by cyclic voltammetry. J Electrochem Soc, 2007, 154: A253
Jin B, Jin E M, Park K H, et al. Electrochemical properties of LiFePO4-multiwalled carbon nanotubes composite cathode materials for lithium polymer battery. Electrochem Commun, 2008, 10: 1537–1540
Li J, Qu Q, Zhang L, et al. A monodispersed nano-hexahedral LiFePO4 with improved power capability by carbon-coatings. J Alloys Compd, 2013, 579: 377–383
Chen J, Zhao N, Li G D, et al. Superior performance of LiFePO4/C with porous structure synthesized by an in situ polymerization restriction method for lithium ion batteries. Mater Chem Phys, 2016, 180: 244–249
Chen K F, Xue D F. Rare earth and transitional metal colloidal supercapacitors. Sci China Tech Sci, 2015, 58: 1768–1778
Liu F, Xue D F. Electrochemical energy storage applications of “pristine” graphene produced by non-oxidative routes. Sci China Tech Sci, 2015, 58: 1841–1850
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, H., Lu, J., Huang, X. et al. Improved electrochemical performance of LiFe0.65Mn0.35PO4 cathode material by using electrolytic manganese dioxide for lithium-ion battery. Sci. China Technol. Sci. 60, 1853–1860 (2017). https://doi.org/10.1007/s11431-017-9153-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11431-017-9153-x