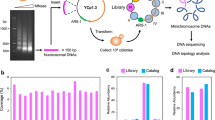
Abstract
Yeast artificial chromosomes (YACs) are important tools for sequencing, gene cloning, and transferring large quantities of genetic information. However, the structure and activity of YAC chromatin, as well as the unintended impacts of introducing foreign DNA sequences on DNA-associated biochemical events, have not been widely explored. Here, we showed that abundant genetic elements like TATA box and transcription factor-binding motifs occurred unintentionally in a previously reported data-carrying chromosome (dChr). In addition, we used state-of-the-art sequencing technologies to comprehensively profile the genetic, epigenetic, transcriptional, and proteomic characteristics of the exogenous dChr. We found that the data-carrying DNA formed active chromatin with high chromatin accessibility and H3K4 tri-methylation levels. The dChr also displayed highly pervasive transcriptional ability and transcribed hundreds of noncoding RNAs. The results demonstrated that exogenous artificial chromosomes formed chromatin structures and did not remain as naked or loose plasmids. A better understanding of the YAC chromatin nature will improve our ability to design better data-storage chromosomes.
Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Data availability
Related sequencing data have been uploaded to NCBI’s Gene Expression Omnibus and are accessible through the GEO Series accession number GSE183492. Other data supporting the findings of the present study are available from the corresponding author upon reasonable request.
References
Adiconis, X., Haber, A.L., Simmons, S.K., Levy Moonshine, A., Ji, Z., Busby, M.A., Shi, X., Jacques, J., Lancaster, M.A., Pan, J.Q., et al. (2018). Comprehensive comparative analysis of 5′-end RNA-sequencing methods. Nat Methods 15, 505–511.
Bailey, T.L., Johnson, J., Grant, C.E., and Noble, W.S. (2015). The MEME Suite. Nucleic Acids Res 43, W39–W49.
Basehoar, A.D., Zanton, S.J., and Pugh, B.F. (2004). Identification and distinct regulation of yeast TATA box-containing genes. Cell 116, 699–709.
Chen, W., Han, M., Zhou, J., Ge, Q., Wang, P., Zhang, X., Zhu, S., Song, L., and Yuan, Y. (2021). An artificial chromosome for data storage. Natl Sci Rev 8, nwab028.
Choi, S.W., Kim, H.W., and Nam, J.W. (2019). The small peptide world in long noncoding RNAs. Brief Bioinform 20, 1853–1864.
Chong, S.Y., Cutler, S., Lin, J.J., Tsai, C.H., Tsai, H.K., Biggins, S., Tsukiyama, T., Lo, Y.C., and Kao, C.F. (2020). H3K4 methylation at active genes mitigates transcription-replication conflicts during replication stress. Nat Commun 11, 809.
Corces, M.R., Trevino, A.E., Hamilton, E.G., Greenside, P.G., SinnottArmstrong, N.A., Vesuna, S., Satpathy, A.T., Rubin, A.J., Montine, K. S., Wu, B., et al. (2017). An improved ATAC-seq protocol reduces background and enables interrogation of frozen tissues. Nat Methods 14, 959–962.
Durand, N.C., Robinson, J.T., Shamim, M.S., Machol, I., Mesirov, J.P., Lander, E.S., and Aiden, E.L. (2016). Juicebox provides a visualization system for Hi-C contact maps with unlimited zoom. Cell Syst 3, 99–101.
Fang, F., Zhao, Q., Chu, H., Liu, M., Zhao, B., Liang, Z., Zhang, L., Li, G., Wang, L., Qin, J., et al. (2020). Molecular dynamics simulation-assisted ionic liquid screening for deep coverage proteome analysis. Mol Cell Proteomics 19, 1724–1737.
Fredens, J., Wang, K., de la Torre, D., Funke, L.F.H., Robertson, W.E., Christova, Y., Chia, T., Schmied, W.H., Dunkelmann, D.L., Beránek, V., et al. (2019). Total synthesis of Escherichia coli with a recoded genome. Nature 569, 514–518.
Gibson, D.G., Glass, J.I., Lartigue, C., Noskov, V.N., Chuang, R.Y., Algire, M.A., Benders, G.A., Montague, M.G., Ma, L., Moodie, M.M., et al. (2010). Creation of a bacterial cell controlled by a chemically synthesized genome. Science 329, 52–56.
Gnügge, R., and Rudolf, F. (2017). Saccharomyces cerevisiae Shuttle vectors. Yeast 34, 205–221.
Gordon, S.P., Tseng, E., Salamov, A., Zhang, J., Meng, X., Zhao, Z., Kang, D., Underwood, J., Grigoriev, I.V., Figueroa, M., et al. (2015). Widespread polycistronic transcripts in fungi revealed by single-molecule mRNA sequencing. PLoS ONE 10, e0132628.
Grant, C.E., Bailey, T.L., and Noble, W.S. (2011). FIMO: scanning for occurrences of a given motif. Bioinformatics 27, 1017–1018.
Hieter, P., Mann, C., Snyder, M., and Davis, R.W. (1985). Mitotic stability of yeast chromosomes: a colony color assay that measures nondisjunction and chromosome loss. Cell 40, 381–392.
HutchisonIII, C.A., Chuang, R.Y., Noskov, V.N., Assad-Garcia, N., Deerinck, T.J., Ellisman, M.H., Gill, J., Kannan, K., Karas, B.J., Ma, L., et al. (2016). Design and synthesis of a minimal bacterial genome. Science 351, aad6253.
Kannan, K., and Gibson, D.G. (2017). Yeast genome, by design. Science 355, 1024–1025.
Keung, A.J., Joung, J.K., Khalil, A.S., and Collins, J.J. (2015). Chromatin regulation at the frontier of synthetic biology. Nat Rev Genet 16, 159–171.
Kim, S., Liachko, I., Brickner, D.G., Cook, K., Noble, W.S., Brickner, J.H., Shendure, J., and Dunham, M.J. (2017). The dynamic three-dimensional organization of the diploid yeast genome. eLife 6, e23623.
Kim, T.H., and Dekker, J. (2018). ChIP-quantitative polymerase chain reaction (ChIP-qPCR). Cold Spring Harb Protoc 2018(5), pdb.prot082628.
Klemm, S.L., Shipony, Z., and Greenleaf, W.J. (2019). Chromatin accessibility and the regulatory epigenome. Nat Rev Genet 20, 207–220.
Knudsen, S. (1999). Promoter2.0: for the recognition of PolII promoter sequences. Bioinformatics 15, 356–361.
Kouprina, N., and Larionov, V. (2008). Selective isolation of genomic loci from complex genomes by transformation-associated recombination cloning in the yeast Saccharomyces cerevisiae. Nat Protoc 3, 371–377.
Lamb, B.T., and Gearhart, J.D. (1995). YAC transgenics and the study of genetics and human disease. Curr Opin Genet Dev 5, 342–348.
Lee, C.S.K., Cheung, M.F., Li, J., Zhao, Y., Lam, W.H., Ho, V., Rohs, R., Zhai, Y., Leung, D., and Tye, B.K. (2021). Humanizing the yeast origin recognition complex. Nat Commun 12, 33.
Lieberman-Aiden, E., van Berkum, N.L., Williams, L., Imakaev, M., Ragoczy, T., Telling, A., Amit, I., Lajoie, B.R., Sabo, P.J., Dorschner, M.O., et al. (2009). Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science 326, 289–293.
Lu, X., and Ellis, T. (2021). Self-replicating digital data storage with synthetic chromosomes. Natl Sci Rev 8.
Lu, Z., and Lin, Z. (2019). Pervasive and dynamic transcription initiation in Saccharomyces cerevisiae. Genome Res 29, 1198–1210.
Luo, X., Reiter, M.A., d’Espaux, L., Wong, J., Denby, C.M., Lechner, A., Zhang, Y., Grzybowski, A.T., Harth, S., Lin, W., et al. (2019). Complete biosynthesis of cannabinoids and their unnatural analogues in yeast. Nature 567, 123–126.
Marschall, P., Malik, N., and Larin, Z. (1999). Transfer of YACs up to 2.3 Mb intact into human cells with polyethylenimine. Gene Ther 6, 1634–1637.
Postma, E.D., Dashko, S., van Breemen, L., Taylor Parkins, S.K., van den Broek, M., Daran, J.M., and Daran-Lapujade, P. (2021). A supernumerary designer chromosome for modular in vivo pathway assembly in Saccharomyces cerevisiae. Nucleic Acids Res 49, 1769–1783.
Pouokam, M., Cruz, B., Burgess, S., Segal, M.R., Vazquez, M., and Arsuaga, J. (2019). The Rabl configuration limits topological entanglement of chromosomes in budding yeast. Sci Rep 9, 6795.
Ro, D.K., Paradise, E.M., Ouellet, M., Fisher, K.J., Newman, K.L., Ndungu, J.M., Ho, K.A., Eachus, R.A., Ham, T.S., Kirby, J., et al. (2006). Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature 440, 940–943.
Sasaki, T., Matsumoto, T., Antonio, B.A., and Nagamura, Y. (2005). From mapping to sequencing, post-sequencing and beyond. Plant Cell Physiol 46, 3–13.
Servant, N., Varoquaux, N., Lajoie, B.R., Viara, E., Chen, C.J., Vert, J.P., Heard, E., Dekker, J., and Barillot, E. (2015). HiC-Pro: an optimized and flexible pipeline for Hi-C data processing. Genome Biol 16, 259.
Sharon, D., Tilgner, H., Grubert, F., and Snyder, M. (2013). A single-molecule long-read survey of the human transcriptome. Nat Biotechnol 31, 1009–1014.
Shen, Y., Wang, Y., Chen, T., Gao, F., Gong, J., Abramczyk, D., Walker, R., Zhao, H., Chen, S., Liu, W., et al. (2017). Deep functional analysis of synII, a 770-kilobase synthetic yeast chromosome. Science 355, aaf4791.
Shiraki, T., Kondo, S., Katayama, S., Waki, K., Kasukawa, T., Kawaji, H., Kodzius, R., Watahiki, A., Nakamura, M., Arakawa, T., et al. (2003). Cap analysis gene expression for high-throughput analysis of transcriptional starting point and identification of promoter usage. Proc Natl Acad Sci USA 100, 15776–15781.
Srinivasan, P., and Smolke, C.D. (2020). Biosynthesis of medicinal tropane alkaloids in yeast. Nature 585, 614–619.
Sylvain, M.A., Liang, X.B., Hellauer, K., and Turcotte, B. (2011). Yeast zinc cluster proteins Dal81 and Uga3 cooperate by targeting common coactivators for transcriptional activation of γ-aminobutyrate responsive genes. Genetics 188, 523–534.
Tkach, J.M., Yimit, A., Lee, A.Y., Riffle, M., Costanzo, M., Jaschob, D., Hendry, J.A., Ou, J., Moffat, J., Boone, C., et al. (2012). Dissecting DNA damage response pathways by analysing protein localization and abundance changes during DNA replication stress. Nat Cell Biol 14, 966–976.
Treutlein, B., Gokce, O., Quake, S.R., and Südhof, T.C. (2014). Cartography of neurexin alternative splicing mapped by single-molecule long-read mRNA sequencing. Proc Natl Acad Sci USA 111, E1291–E1299.
Tschumper, G., and Carbon, J. (1983). Copy number control by a yeast centromere. Gene 23, 221–232.
Tudek, A., Candelli, T., and Libri, D. (2015). Non-coding transcription by RNA polymerase II in yeast: Hasard or nécessité? Biochimie 117, 28–36.
Wang, K., Fredens, J., Brunner, S.F., Kim, S.H., Chia, T., and Chin, J.W. (2016). Defining synonymous codon compression schemes by genome recoding. Nature 539, 59–64.
Wang, R., Mozziconacci, J., Bancaud, A., and Gadal, O. (2015). Principles of chromatin organization in yeast: relevance of polymer models to describe nuclear organization and dynamics. Curr Opin Cell Biol 34, 54–60.
Wilkinson, D., Váchová, L., Hlaváček, O., Maršíková, J., Gilfillan, G.D., and Palková, Z. (2018). Long noncoding RNAs in yeast cells and differentiated subpopulations of yeast colonies and biofilms. Oxid Med Cell Longev 2018, 1–12.
Wu, Y., Li, B.Z., Zhao, M., Mitchell, L.A., Xie, Z.X., Lin, Q.H., Wang, X., Xiao, W.H., Wang, Y., Zhou, X., et al. (2017). Bug mapping and fitness testing of chemically synthesized chromosome X. Science 355, eaaf4706.
Xie, Z.X., Li, B.Z., Mitchell, L.A., Wu, Y., Qi, X., Jin, Z., Jia, B., Wang, X., Zeng, B.X., Liu, H.M., et al. (2017). “Perfect” designer chromosome V and behavior of a ring derivative. Science 355, aaf4704.
Young, M.D., Wakefield, M.J., Smyth, G.K., and Oshlack, A. (2010). Gene ontology analysis for RNA-seq: accounting for selection bias. Genome Biol 11, R14.
Zhang, C., Xu, Z., Yang, S., Sun, G., Jia, L., Zheng, Z., Gu, Q., Tao, W., Cheng, T., Li, C., et al. (2020). tagHi-C reveals 3D chromatin architecture dynamics during mouse hematopoiesis. Cell Rep 32, 108206.
Zhang, W., Zhao, G., Luo, Z., Lin, Y., Wang, L., Guo, Y., Wang, A., Jiang, S., Jiang, Q., Gong, J., et al. (2017). Engineering the ribosomal DNA in a megabase synthetic chromosome. Science 355, eaaf3981.
Zhao, Q., Fang, F., Shan, Y., Sui, Z., Zhao, B., Liang, Z., Zhang, L., and Zhang, Y. (2017). In-depth proteome coverage by improving efficiency for membrane proteome analysis. Anal Chem 89, 5179–5185.
Zhou, J., Wu, R., Xue, X., and Qin, Z. (2016). CasHRA (Cas9-facilitated Homologous Recombination Assembly) method of constructing megabase-sized DNA. Nucleic Acids Res 44, e124.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2121YFA0909300), the National Natural Science Foundation of China (31861143017, 21621004, and 31901019) and the China Postdoctoral Science Foundation (2021M692389). We thank Prof. Yan Zhang at Tianjin University for the manuscript revision.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Compliance and ethics The author(s) declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhou, J., Zhang, C., Wei, R. et al. Exogenous artificial DNA forms chromatin structure with active transcription in yeast. Sci. China Life Sci. 65, 851–860 (2022). https://doi.org/10.1007/s11427-021-2044-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11427-021-2044-x