Abstract
One elusive aspect of the chromosome architecture is how it constrains the DNA topology. Nucleosomes stabilise negative DNA supercoils by restraining a DNA linking number difference (∆Lk) of about −1.26. However, whether this capacity is uniform across the genome is unknown. Here, we calculate the ∆Lk restrained by over 4000 nucleosomes in yeast cells. To achieve this, we insert each nucleosome in a circular minichromosome and perform Topo-seq, a high-throughput procedure to inspect the topology of circular DNA libraries in one gel electrophoresis. We show that nucleosomes inherently restrain distinct ∆Lk values depending on their genomic origin. Nucleosome DNA topologies differ at gene bodies (∆Lk = −1.29), intergenic regions (∆Lk = −1.23), rDNA genes (∆Lk = −1.24) and telomeric regions (∆Lk = −1.07). Nucleosomes near the transcription start and termination sites also exhibit singular DNA topologies. Our findings demonstrate that nucleosome DNA topology is imprinted by its native chromatin context and persists when the nucleosome is relocated.
Similar content being viewed by others
Introduction
High-throughput analyses increasingly improve our knowledge of chromatin structure and function genome-wide1,2. However, a fundamental trait that remains elusive to current technologies is the topology of the chromatinized DNA. We do not know how the DNA double helix is twisted and bent along chromatin. We define “constrained topolome” as DNA deformations stabilized by chromatin, and “unconstrained topolome” as those resulting from the mechanical stress the DNA undergoes during genome activities3,4,5.
The principal actor of the constrained topolome in eukaryotic cells is the nucleosome, the DNA packaging unit of chromatin, in which about 147 base pairs (bp) of DNA make nearly 1.6 left-handed super-helical turns around a histone octamer6. However, this canonical configuration is neither uniform nor static7,8,9. The DNA nucleotide sequence, histone composition and modifications affect nucleosome conformation and stability10,11,12. So far, numerous studies have mapped nucleosome structure and position in model organisms, such as budding yeast13,14,15,16,17. However, no study has yet addressed how nucleosomes constrain the topology of DNA throughout the genome.
In a previous study, we developed a strategy to measure the DNA linking number difference (ΔLk) restrained by nucleosomes in vivo18. We compared by DNA electrophoresis the ∆Lk constrained by yeast circular minichromosomes before and after inserting an additional nucleosome. By doing so, we found that not all nucleosomes restrain ∆Lk (DNA supercoils) to the same extent, being the average value of ΔLk = −1.2618. Our next goal was to determine the ∆Lk constrained by all individual nucleosomes. However, since it was unfeasible to perform thousands of electrophoretic analyses (one for each nucleosome), we needed another strategy to circumvent this problem.
Here, we develop a high-throughput procedure termed “Topo-seq” to analyse the topology of large libraries of circular DNA molecules. We apply Topo-seq to a library of 4000 nucleosomes hosted in yeast circular minichromosomes in vivo. Our results reveal that nucleosomes inherently restrain distinctive ∆Lk values depending on their genomic origin. Therefore, nucleosome DNA topology is an intrinsic trait imprinted by the native chromatin context, which persists when allocated somewhere else. We discuss the implications of this nucleosomal feature and the potential of Topo-seq to disclose further DNA topology genome-wide.
Results
Construction of minichromosomes hosting a nucleosome DNA library
As in our previous study18, we digested budding yeast chromatin with Micrococcal nuclease and purified a pool of mono-nucleosomal DNA fragments (≈ 150 bp) that combined increasing digestion rates (Fig. 1a). After repairing the DNA ends, we added adaptors to insert the nucleosome DNA library in the YCp1.3 (1341 bp) yeast circular minichromosome (Supplementary Fig. 1). Next, we introduced these minichromosomes, each containing one nucleosome of the library, into yeast cells and obtained about ten thousand transformants. We pooled all these colonies and extracted their DNA (Fig. 1a). Parallel DNA sequencing of the minichromosomes revealed a library of 8369 mono-nucleosome DNA fragments of length 144 ± 21 bp (mean ± SD). Nearly all (> 91%) of these DNA fragments overlapped (> 50 bp) with the genomic coordinates of 4276 previously referenced nucleosomes13 (Supplementary Data 1, Supplementary Fig. 2). The chromosomal distribution (Fig. 1b), genomic type (Fig. 1c), stability (Fig. 1d) and positioning (Supplementary Fig. 3) of the identified nucleosomes were comparable to that of the bulk of referenced nucleosomes13, signifying the nucleosome DNA library was representative.
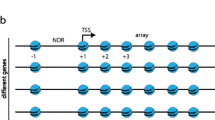
a The gel shows yeast chromatin digested with increasing amounts of Micrococcal nuclease. Nucleosome DNA fragments of about 150 bp (pink) generated in a single experiment with increasing degrees of digestion were pooled and inserted into the YCp1.3 minichromosome DNA (1341 bp) to transform yeast cells. About 104 transformants were collected and pooled for sequencing and topology analysis of the minichromosome DNAs. The allocation of the nucleosome DNA library (N) into the chromatin structure of YCp1.3 is illustrated (see Supplementary Fig. 1 for details). b Coverage (%) of the nucleosome DNA library (see Supplementary Data 1 for nucleosome coordinates) across the 16 yeast chromosomes (I-XVI) normalized per kb. c Relative abundance of nucleosomes from different positions relative to the transcription start site (−1, +1 > + 1) and the transcription terminal site. d Relative abundance of fuzzy and stable nucleosomes. In (c) and (d), the nucleosome DNA library (n = 4276, pink) is compared to the full catalogue of yeast nucleosomes (n = 61110, blue). Source data are provided as a Source data file.
Conceptualization leading to Topo-seq
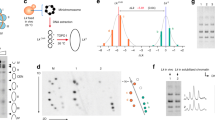
Due to DNA thermodynamics, covalently closed circular DNA molecules present a Gaussian distribution of DNA linking number (Lk) topoisomers, visualized as a ladder of DNA bands by agarose gel electrophoresis19. When the mean Lk value of a DNA molecule relaxed in vitro (Lk0) is compared to that of the DNA in a circular minichromosome (LkChr), the Lk difference is the ∆Lk constrained by the minichromosome chromatin (Lk0 − LkChr = ∆LkChr). Upon adding a new nucleosome to such minichromosome, ∆LkChr changes to ∆LkChr+nuc and the resulting difference (∆LkChr+nuc − ∆LkChr = ∆Lknuc) equals the ∆Lk restrained by the new nucleosome (Supplementary Fig. 4). Following this strategy, we previously showed that most nucleosomes restrain ∆Lk values of about −1.26 in vivo18. However, this strategy was unfeasible for determining the ∆Lk of each nucleosome of our library, as it would require running thousands of electrophoresis. To circumvent this problem, we ran instead all minichromosome DNAs containing the library in a single electrophoresis lane (Fig. 2a, lane 1). As a result, the pool of Lk distributions overlapped and produced a smear rather than a ladder of Lk topoisomers. This smear occurred because the nucleosome sequences of the library were not of equal length (144 ± 21 bp) and, since these length differences were not multiples of the helical repeat of DNA (10.5 bp), most ladders of Lk topoisomers had different phasing. We evidenced this misalignment by running, in the same gel, the Lk distributions of individual minichromosomes that carried nucleosome DNA sequences differing by only 1 to 5 bp in length (Fig. 2a, lanes 2–7). Note that Lk distributions can have equal or distinct Lk mean (red lines) irrespectively of whether their Lk topoisomers are aligned (Fig. 2b, lanes 2–7). This gel electrophoresis also evidenced that the mean Lk of the pool of Lk distributions produced by the nucleosome DNA library (Fig. 2b, lane 1) virtually matched with that produced by individual nucleosomes that restrained ∆Lk −1.26 (Fig. 2b, lanes 2–5). As in our previous study18, this observation denoted that most fragments of the DNA library assembled nucleosomes and that the average ∆Lk constrained by them is −1.26. Importantly, this value differs markedly from the average ∆Lk of −0.84 constrained by an analogous library of non-nucleosomal (prokaryotic) DNA fragments (Supplementary Fig. 5), corroborating thereby that the average ∆Lk of −1.26 is specific to nucleosomal DNA.
a Gel electrophoresis of Lk distributions. Lane 1, pool of 104 minichromosome DNAs hosting the nucleosome DNA library. Lanes 2–7, individual minichromosome DNAs hosting a nucleosome with the indicated DNA length (bp). Electrophoresis conditions are described in Methods. N, nicked circles. Lk, topoisomers. b Gel densitometry (left, in blue) and relative topoisomer intensities (right, in orange) of the previous Lk distributions (lanes 2–7), indicating their Lk mean position (red lines), standard deviation (SD) and the ∆Lk restrained by the hosted nucleosome (∆Lknuc) calculated as described in Supplementary Fig 4. c Cutting the Lk distributions of sample 6 (∆Lknuc = −1.02) and 7 (∆Lknuc = −1.49) at the global Lk mean position (∆Lknuc =−1.26) produces unequal topoisomer DNA abundances at each side of the cut (orange and blue). As Lk distributions are Gaussian, the distinct partition probabilities of DNA abundance translate into Z-scores (Z) of a normal probability distribution. These Z-scores reflect how far the Lk mean of each distribution is from that of the global average (the cut site), where ∆Lknuc = −1.26. Source data are provided as a Source data file.
At this point, we realized that within the library pool of Lk distributions (Fig. 2b, lane 1), those from nucleosomes restraining ∆Lk <−1.26 and >−1.26 should respectively have their Lk mean slightly ahead and behind the global average (for example, samples 6 and 7 in Fig. 2b). Accordingly, upon cutting the smear of Lk distributions at the level of the global mean, nucleosome DNA sequences restraining ∆Lk <−1.26 and >−1.26 would differently enrich at each side of the cut (Fig. 2c, top). The partition probability of each nucleosome DNA sequence would reflect how far its ∆Lknuc deviates from the global mean (∆Lknuc = −1.26, at the cut site); and, since Lk distributions are Gaussian, this deviation would correlate to the Z-score of a normal distribution (Fig. 2c, bottom). In this way, we could classify all nucleosomes of the library by their capacity to restrain ∆Lk. We termed this procedure “Topo-seq” since it relies on sequencing DNA topoisomers eluted from different sections of an electrophoresis gel.
Topo-seq and calculation of the ∆Lk restrained by nucleosomes
We cut into two sections the gel slab comprising the overlapping Lk distributions of our nucleosome DNA library (Fig. 3a), such that the top section (A) enriched nucleosome DNA sequences restraining ∆Lk >−1.26 and the bottom section (B) those restraining ∆Lk <−1.26. We eluted the DNA from both sections, amplified the nucleosome DNAs by PCR and sequenced them. In this step, note that since ∆Lknuc calculations derive from the partition ratio of each sequence in these gel sections, potential biases due to the length and bp composition of nucleosomal DNAs would similarly occur on both sides and thus have little effect on the partition ratios. As expected, all DNA sequences of the nucleosome DNA library were present in both sections but with distinct partition probabilities (Fig. 3b). Section B was enriched in short nucleosomal DNA sequences (< 140 bp) since the Lk distributions of the corresponding DNA circles migrated faster during electrophoresis. However, the partition ratios A/B varied up to a factor of 10 within each length group, which denoted a diversity in nucleosome DNA topology (Fig. 3c).
a Splitting of the Lk distributions of lane 1 in Fig. 2a at the level of the global Lk mean (∆Lknuc = −1.26); and PCR products of the nucleosome sequences eluted from section A and B. b Plot of the abundance of individual nucleosome DNA sequences in sections A and B. c Plot of the partition probability of each nucleosome DNA sequence (ratio A/B) vs the sequence length (bp). d Plot of the partition probabilities converted into Z-scores of a normal distribution and multiplied by 0.85 (SD of Lk distributions). e Corrected ∆Lk scores after subtracting the average ∆Lk of all the sequences of the same length. f Plot of ∆Lk values after adding or subtracting 0.007 units for each bp difference from 144 bp and finally adding −1.26. See Supplementary Data 2 for calculated values. g Histogram of ∆Lk values restrained by the nucleosome DNA library (mean = −1.26, SD = 0.33). Analyses in (a–f) were conducted once with the nucleosome library (n = 8369) described in Fig. 1. h Models of restrained ∆Lk as a function of the number of DNA super-helical turns (N), considering ∆Tw = +0.2 and ∆Wr = N(1–sin 4o). i Models of restrained ∆Lk as a function of the pitch angle of DNA super-helical turns (∂), considering ∆Tw = +0.2 and ∆Wr = 1.56(1–sin ∂). j Twisting angle (red arrows) at the entry and exit of DNA to produce ∆Tw of 0, +0.2, +0.4 and the indicated ∆Lk, considering ∆Wr = 1.56(1–sin 4o). See Supplementary Fig. 10 for detailed correlations of ∆Wr and ∆Tw on ∆Lk. Source data are provided as a Source data file.
To calculate the ∆Lk restrained by each nucleosome, we converted the partition probability of each nucleosome DNA sequence into a Z-score of a normal distribution of mean zero and a standard deviation one. Next, we multiplied these Z-scores by 0.85, which is the standard deviation of the Lk distributions of the ≈1.5 kb minichromosome DNAs hosting the nucleosome DNA library (Fig. 2b). The resulting values indicated how far (∆Lk units) the mean of each Lk distribution was from the global Lk average (∆Lknuc of −1.26 at the cut site) (Fig. 3d). Next, we adjusted these ∆Lk scores by taking into account that the nucleosome DNA sequences had different lengths. Firstly, to correct the effect of misaligned Lk ladders (Supplementary Fig. 6), we subtracted from each ∆Lk score the average ∆Lk of all the sequences of the same length (i.e., aligned ladders). These corrected ∆Lk values now reflected how the topology of each nucleosome compared to those of equal length (Fig. 3e). Secondly, we calculated that the overall electrophoretic velocity of the Lk distributions shifted the equivalent of 0.007 Lk units for each bp difference in length (Supplementary Fig. 7). Therefore, we adjusted the ∆Lk values by adding or subtracting 0.007 for each bp difference from 144 bp. Finally, as the current ∆Lk values were relative to the global average (∆Lknuc = −1.26), we added −1.26 to obtain the actual ∆Lk restrained by each nucleosome (Fig. 3f, Supplementary Data 2).
Conversion of ∆Lknuc into twist and writhe deformations of DNA
The ∆Lknuc values calculated via Topo-seq (mean −1.26, SD 0.33) denoted a substantial heterogeneity in the nucleosome DNA topology (Fig. 3g). This heterogeneity did not correlate with the extent of overlap of our library with previously referenced nucleosomes (Supplementary Fig. 8); and it was neither consequent to variability in DNA linker length, which we tested by examining the ∆Lknuc of a nucleosome with different linker sizes (Supplementary Fig. 9 and Supplementary Table 1). Therefore, since ∆Lk = ∆Tw + ∆Wr, nucleosomes with distinct ∆Lknuc values could differ in restraining ∆Tw (helical twist) but, more likely, by their capacity to constrain ∆Wr (super-helical turns). We then modelled how ∆Wr values would translate into ∆Lk by considering ∆Tw = +0.2 (as in the canonic nucleosome) and ∆Wr = N(1–sin ∂)18, where N is the number of wrapped super-helical turns (Fig. 3h, Supplementary Fig. 10a) and ∂ their pitch angle (Fig. 3i, Supplementary Fig. 10b). We also considered how ∆Tw values would translate into ∆Lk, being ∆Wr = −1.46 (Fig. 3j, Supplementary Fig. 10c). For simplicity, we depicted only symmetric shapes, but asymmetric changes of ∆Wr and ∆Tw are likely to occur and combine producing a broad spectrum of possible conformations even for nucleosomes restraining similar ∆Lk values.
Correlation of ∆Lknuc with the composition of nucleosome DNAs
We examined whether the nucleosome capacity to restrain ∆Lk would depend on the DNA nucleotide composition, which affects DNA flexibility and curvature20. We found that GC content was significantly higher (p < 2e−12) for nucleosomes restraining ∆Lk > −1.26 (GC 42.3%) compared to those of ∆Lk < −1.26 (GC 40.7%) (Fig. 4a). Regarding dinucleotide frequencies, nucleosomal DNAs of the library were enriched in AA/TT/TA dinucleotides, being CG/GG/GC dinucleotides the less abundant. However, this general trend faded in the nucleosomes restraining less negative ∆Lk values, which exhibited an abundance of other dinucleotides such as CA/TG (Fig. 4b). As expected, AA/TT/TA dinucleotides had a periodical spacing close to the DNA helical repeat (10–11 nts), though nucleosomes restraining less negative ∆Lk values also presented other dinucleotides with short periodic patterns (Fig. 4c). Since DNA composition affects the position stability of nucleosomes21,22, we examined whether the restrained ∆Lk correlated to nucleosome stability. We found that the positional fuzziness of nucleosomes, measured in their natural loci13, had no relation with their intrinsic capacity to constrain ∆Lk (Fig. 4d).
a GC content of nucleosomal DNAs of the library with ∆Lknuc values <−1.26 (n = 4187) and >−1.26 (n = 3611). In the box plots, the centre line denotes the median value, while the box marks the 25th (upper limit) to 75th (lower limit) percentiles of the dataset. The black whiskers mark the 5th and 95th percentiles. A p-value of 1.8 E−12 was determined by a two-sided Wilcoxon test. b Heatmap of dinucleotide frequencies for ∆Lknuc values of the library (n = 8369) in 5 quantiles. c Heatmap of dinucleotide periodicities for ∆Lknuc values of the library (n = 8369) in 10 quantiles. d Scatter plot of ∆Lknuc values of the library (n = 8369) against the native positional fuzziness of nucleosomes. Source data are provided as a Source data file.
Correlation of ∆Lknuc with the genomic origin of nucleosomes
Next, we examined whether the nucleosome capacity to restrain ∆Lk would depend on their genomic origin. In this case, several unexpected correlations emerged (Fig. 5, Supplementary Data 3). Gene body nucleosomes constrained a mean ∆Lknuc of −1.29, which was distinct (p < 0.001) from the mean ∆Lknuc of −1.23 constrained by intergenic nucleosomes (Fig. 5a). The ∆Lk restrained by gene body nucleosomes (transcribed by Pol-II) was also different (p < 0.004) from the mean ∆Lknuc of −1.24 restrained by nucleosomes of rDNA genes (transcribed by Pol-I and -III) (Fig. 5a). Within gene body nucleosomes, there were also significant differences depending on the nucleosome position relative to the transcription start site (TSS) and termination site (TTS) (Fig. 5b). Nucleosomes at position −1 from TSS restrained a mean ∆Lknuc of −1.27, a value more similar to gene body than intergenic nucleosomes. Nucleosomes at position +1, stably positioned downstream of the TSS, also restrained a mean ∆Lknuc of −1.27, like the bulk of gene body nucleosomes. However, two gene body nucleosomes constrained ∆Lk values significantly more negative than the others. Nucleosomes at position +2 restrained a mean ∆Lknuc of −1.33 (p < 0.001), and terminal nucleosomes at the TTS restrained a mean ∆Lknuc of −1.32 (p < 0.008). Lastly, Topo-seq uncovered that the nucleosomes from telomeric regions23 were those with the most atypical DNA topology. These nucleosomes presented short DNA sequences (136 bp on average) and a mean ∆Lknuc of −1.07, highly deviated (p < E−60) from the mean ∆Lknuc of −1.27 of non-telomeric nucleosomes (Fig. 5c). The topology of telomeric nucleosomes was significantly distinct even when compared to short nucleosomal DNAs of non-telomeric regions (Supplementary Fig. 11).
a ∆Lk restrained by gene body (n = 4460), intergenic (n = 2495) and rDNA nucleosomes (n = 2043). b ∆Lk restrained nucleosomes at positions −1 (n = 342), + 1 (n = 403), + 2 (n = 787), +3 to +5 (n = 1142) relative to the TSS and the TTS (Term). c ∆Lk restrained by non-telomeric (n = 7673) and telomeric nucleosomes (n = 696). In (a–c) box plots, the centre line denotes the median value, while the box marks the 25th (upper limit) to 75th (lower limit) percentiles of the dataset. The black whiskers mark the 5th and 95th percentiles. In (a–b), p-values were determined by two-sided ANOVA with Tukey’s test. In (c), the p-value was determined by an unpaired two-sided t-test. The bottom rows in (a–c) indicate the number (n), average sequence length (bp) and mean ∆Lk of nucleosomes in each class. The ∆Lknuc and genomic allocation of individual nucleosomes are described in Supplementary Data 3. d Correlation of ∆Lknuc values obtained via Topo-seq with those calculated via the analyses of individual Lk distributions (see Supplementary Fig 12). The scatter plot shows the Lknuc of 18 representative nucleosomes (3 gene body, 3 intergenic, 3 rDNA, 3 telomeric, 3 nuc+2, 3 terminal) obtained by both procedures. Nucleosome coordinates and ∆Lknuc values are specified in Supplementary Table 1. Source data are provided as a Source data file.
Validation of the ∆Lknuc dependence on the origin of nucleosomes
To validate the location-dependent differences of ∆Lknuc uncovered by Topo-seq, we measured the ∆Lknuc of individual nucleosomes of each genomic region. We chose 18 nucleosomes (3 gene boby, 3 intergene, 3 rDNA, 3 telomeric, 3 nuc+2, 3 terminal), whose ∆Lknuc value determined via Topo-seq was representative of the mean ∆Lknuc of their corresponding region (Supplementary Table 1). We amplified and inserted these sequences (147 bp in length) in the YCp1.3 plasmid, transformed yeast cells and examined the individual Lk distributions of the resulting minichromosomes (Supplementary Fig. 12). The ∆Lknuc values of these 18 nucleosomes presented a good correlation (R2 ≈ 0.9) with the ∆Lknuc values calculated via Topo-seq (Fig. 5d). These results confirmed that the intrinsic capacity of nucleosomes to restrain DNA supercoils depends on their genomic origin; and proved that Topo-seq is a faithful high-throughput procedure to examine the topology of circular DNA libraries.
Discussion
Our study provides two advances toward the unravelling of intracellular DNA topology. First, it shows the feasibility of Topo-seq, a high-throughput procedure for examining topolomes. Second, it demonstrates that DNA retains a topological memory of its deformation by chromatin.
To date, gel electrophoresis of circular DNA molecules is the only procedure to quantitatively characterize changes in the linking number (supercoiling) and the degree of entanglement (knotting or catenation) of DNA. Therefore, to examine local DNA topology within cellular chromosomes, one strategy has been popping out DNA rings at specific chromatin regions24,25,26. However, this procedure would be extremely tedious, if not impracticable, for conducting genome-wide analyses of DNA topology. Topo-seq relies on the sequencing of DNA topoisomers eluted from distinct sections of one gel electrophoresis and, therefore, permits inspecting the topology of multiple DNA molecules at once. We show that DNA amounts as little as 0.1 ng, comprising a library of thousands of DNA constructs, can be eluted from an agarose gel and processed for high-throughput sequencing. Since the calculation of ∆Lk values derives from the partition probability of each sequence in different gel sections, these ratios can be calculated regardless of the abundance of each sequence in the initial library pool. In the present study, since the circular DNAs containing the nucleosome DNA library were of similar length (about 1.5 kb), we resolved the pool of ∆Lk distributions in a one-dimensional gel and then cut it into two sections at the level of the global Lk mean. We then applied corrections for the little length differences, which effect on DNA migration was always smaller than the gel distance between individual Lk topoisomers. However, for future developments of Topo-seq, DNA libraries containing constructs of multiple lengths (1 to 15 kb) could be analysed by cutting the gel into many small sections such that the position and abundance of any DNA topoisomer could be determined irrespective of its size. Another possibility is running the DNA library in a two-dimensional gel. In this case, Lk distributions would resolve into a diagonal of arched ladders, such that Lk topoisomers from one DNA length do not overlay with those of another.
After calculating the ∆Lk values constrained by our nucleosome DNA library, we found structural and functional correlations that validated the reliability of Topo-seq. Importantly, since we interrogated the nucleosomes outside their native genomic locus, the restrained ∆Lk values had to rely on the intrinsic traits of their DNA sequences. In this respect, since high GC content makes the DNA stiffer27,28, the increased GC content of nucleosomes restraining less negative ∆Lk values might reflect a reduced capacity of DNA to completely or stably wrap around histone cores. As expected, the nucleosome DNA library exhibited a 10–11 bp periodicity of AA/TT/TA dinucleotides, which is known to favour nucleosome positioning and stability21,22. But, curiously, this dinucleotide pattern did not correlate with the capacity to restrain ∆Lk. Only the nucleosomes constraining less negative ∆Lk values presented atypical dinucleotide periodicities, which emerged from the repetitive DNA sequences of telomeric regions, as discussed below. Therefore, nucleosome DNA topology might not only depend on dinucleotide parameters, which are also weak predictors of the intrinsic cyclability of DNA29. Moreover, the no correlation between the nucleosome capacity to restrain ∆Lk and their native genomic stability also denoted these are independent structural traits. Conceivably, positional stability depends on the central turn of DNA around the H3-H4 tetramer; and DNA topology mainly relies on interactions with the H2A-H2B dimers configuring the geometry of nucleosome entry and exit DNA segments.
In contrast to the nucleotide composition, the genomic origin of nucleosomes presented striking correlations to their intrinsic capacity to restrain ∆Lk (Fig. 6). The gene body nucleosomes (mean ∆Lknuc of −1.29) are distinct from the intergenic ones (mean ∆Lknuc of −1.23). If this difference relies on the extent of DNA wrapping (∆Wr) and the subsequent different orientations of DNA entry and exit segments, nucleosome arrays in genic and intergenic regions must adopt distinct architectures. Interestingly, this inference might relate to the distinctive folding motifs described in yeast chromatin, where the gene body nucleosomes tend to present tetrahedron folds and the intergenic ones rhomboidal folds16 (Fig. 6). The distinct DNA topology of gene body and intergenic nucleosomes could also reflect that the firsts are suited to confront the torque generated during DNA transcription30 and to facilitate DNA tearing by transcribing RNA polymerases31. However, the topology of gene body nucleosome is not observed in the rDNA genes (mean ∆Lknuc of −1.24). Since rDNA nucleosomes are evicted by the transcribing trains of RNA polymerase I32,33, their distinctive DNA topology probably reflects this unique biophysical context, quite different from genes transcribed by RNA polymerase II.
The ∆Lknuc values of nucleosomes are interpreted and modelled as different capacities to wrap the DNA (brown) around a histone core (blue). Tetra-nucleosomes illustrate chromatin folding motifs proposed for gene bodies (tetrahedron), intergenic regions (rhomboidal) and telomeric chromatin (columnar).
Within gene body nucleosomes, we expected nucleosomes at positions −1 and +1 relative to the TSS would have singular DNA topologies since they delimit a nucleosome-free region (NFR) in most gene promoters17,34,35. Their position relies on transcription factors and chromatin remodelling activities that push them out of the NFR36,37,38,39. The +1 nucleosomes also interact with the transcription preinitiation complex (PIC)40,41. However, the intrinsic capacity of the −1 and +1 nucleosomes to restrain ∆Lk is similar to that of most gene body ones. In contrast, Topo-seq uncovered singular DNA topologies for nucleosomes at positions +2 and the TTS. The case of the +2 nucleosomes (∆Lknuc = −1.33) is striking because they presented DNA sequences shorter (138,3 bp) than the global average and still restrained more negative ∆Lk values than any other nucleosome. The +1 and +2 nucleosomes have high positional stability35 and are recognized as a dinucleosome by remodelling complexes36,42. This feature and a plausible role in the promoter-proximal pausing of RNA Polymerase II43,44 might explain the singular topology of +2 nucleosomes. Regarding the TTS nucleosomes, their high capacity to restrain DNA supercoils (∆Lknuc = −1.32) likely relates to their role as transcription elongation barriers. Interestingly, their capacity to mark transcription termination depends on a proper configuration of their DNA entry-exit site and, thus, their DNA topology45.
Lastly, Topo-seq uncovered nucleosomes from telomeric regions23 as those with the most unusual DNA topology (∆Lknuc = −1.07). Remarkably, these nucleosomes presented shorter DNA sequences (1363 bp) than the non-telomeric ones; and exhibited unusual patterns of dinucleotides since they host abundant short DNA sequence repeats46. Therefore, their reduced capacity to restrain ∆Lk likely reflects an incomplete or distinctive wrapping of the DNA (Fig. 6). Their peculiar DNA topology might relate to the recently reported columnar architecture of telomeric chromatin47 that shows nucleosomes stacked on each other with a repeat length of about 132 bp, letting the DNA wound as a continuous superhelix (Fig. 6).
Taken together, the correlations of ∆Lknuc with the genomic origin of nucleosomes demonstrate that some aspects of the native chromatin context are imprinted on the topology of nucleosomal DNA, such that they reverberate when nucleosomes are placed outside their natural genomic loci. In the same way that dinucleotide periodicity has been optimized for wrapping nucleosomal DNA around histone cores, other traits of the nucleosomal DNA sequences might have been adjusted to adapt nucleosomes to specific chromatin configurations or genomic environments. Therefore, since the intrinsic topology of nucleosomal DNA retains a memory of its native allocation, this inherent trait of nucleosomes becomes a new determinant of chromatin architecture. However, note that the ∆Lknuc values measured in the minichromosome hosting the nucleosome DNA library are not necessarily equal to the ∆Lknuc of the nucleosomes in their native allocation, where each nucleosome interplays with its corresponding chromatin environment. Likewise, note that the ∆Lknuc values of some nucleosomal DNA sequences could be also altered by the recruitment of additional DNA binding factors, nucleosome remodelling activities or reflect partial nucleosome occupancy. We foresee that applying Topo-seq to more extensive libraries of chromatin elements, in combination with chromatin immunoprecipitation or other biochemical selectors of chromatin composition, will provide unprecedented insights into the interplay of chromatin and DNA topology genome-wide. Ultimately, the need for circular minichromosomes to host these libraries could be dispensable if, for instance, the bulk of intracellular chromatin is fragmented and circularised in situ. In this case, upon running an entire genomic pool of DNA rings in a single gel electrophoresis, Topo-seq could readily disclose the topolome of any cell type.
Methods
Construction of the nucleosome DNA library
Saccharomyces cerevisiae FY251 cells (MATa his3-∆200 leu2-D1 trp1-∆63 ura3–52) were grown at 28 °C in 250 ml of rich medium until OD 1.0. Cells were collected, washed with water, and incubated with 80 ml of 1 M Sorbitol, 30 mM DTT for 15 min at 28 °C. Next, 625 U of Lyticase (Sigma-Aldrich L2524) and 10 μL of 4 M NaOH were added to the cell’s suspension, and the incubation continued until >80% of cells converted into spheroplasts. Spheroplasts were washed with 1 M Sorbitol and resuspended in 1.5 ml of hypotonic lysis buffer (1 mM CaCl2 5 mM KH2PO4 1 mM PMSF) at 24 °C. After adding 30 units of micrococcal nuclease (Sigma-Aldrich N3755), the lysate was incubated at 24 °C. Aliquots of 300 µl were quenched with 20 mM EDTA 1% SDS at different incubation times (3 to 30 min). Gel electrophoresis of digested chromatin was done in 1% agarose in TBE buffer, at 80 V for 3 h. Mono-nucleosome DNA fragments (about 150 bp in length) produced at successive digestion times were gel-eluted and pooled. The DNA ends produced by micrococcal nuclease were repaired by removing terminal 3’-phosphates with T4-polynucleotide kinase and filled with Klenow and T4-DNA polymerase activities. The resulting A-tailed DNA fragments were ligated to T-tailed adaptors that provided degenerated Asc1 or BamH1 ligation ends (see Supplementary Fig. 1). The adapted DNA fragments were inserted between the Asc1 and BamH1 sites of the YCp1.3 DNA (1341 bp), which was hosted in a bacterial plasmid. After amplifying these plasmids in E. coli cells, they were digested with Not1 to release DNA segments of about 1.55 kb that comprised YCp1.3 with the inserted library of mono-nucleosome DNAs (≈ 200 bp). These DNA segments were gel purified and circularised by ligating their Not1 ends.
Extraction of minichromosome DNAs hosting the library
Monomeric circles of YCp1.3 hosting the nucleosome DNA library were gel-purified and used to transform FY251 via electroporation. About ten thousand colonies were collected from agar plates containing yeast synthetic media (TRP dropout). These colonies were pooled, washed with water, resuspended in 200 ml of TRP dropout and incubated at 28 °C for 2 h. The cells were then fixed by mixing the suspension with one cold volume (−20 °C) of ET solution (Ethanol 95%, Toluene 28 mM, Tris HCl pH 8.8 20 mM, EDTA 5 mM). The cells were sedimented at room temperature, washed twice with water, resuspended in 400 µl of TE, and transferred to a 1.5-ml microfuge tube containing 400 µl of phenol and 400 µl of acid-washed glass beads (425–600 µm, Sigma). Mechanic lysis of >80% cells was achieved by shaking the tubes in a FastPrep® apparatus for 10 s at power 5. The aqueous phase of the lysate was collected, extracted with chloroform, precipitated with ethanol, and dissolved in 100 µl of TE containing RNAse-A. After a 15-min incubation at 37 °C, the samples were extracted with phenol and chloroform, the DNA precipitated with ethanol and dissolved in 50 µl of TE.
Construction of minichromosomes hosting individual nucleosomes
Individual nucleosomal DNA sequences of specific length were obtained from yeast genomic DNA via PCR amplification (NEB Taq Polymerase) by using the primers described in Supplementary Table 1. The resulting A-tailed DNA fragments were ligated to T-tailed adaptors to be inserted between the Asc1 and BamH1 sites of the YCp1.3 DNA, as described above. Yeast cells transformed with minichromosomes hosting the individual nucleosomes were grown in 20 ml of TRP dropout at 28 °C. When the cultures reached the exponential phase (OD ≈ 1), the cells were fixed and the minichromosome DNAs were extracted as described above.
Electrophoresis of Lk distributions
The DNA of minichromosomes that hosted individual nucleosomes or the nucleosome DNA library was loaded onto 1.4% (w/v) agarose gels. Electrophoreses were carried out at 2.5 V/cm for 18 h in TBE buffer (89 mM Tris-borate and 2 mM EDTA) containing 0.55 µg/ml chloroquine. Gels were blot-transferred to a nylon membrane and probed at 60 °C with the YCp1.3 DNA labelled with AlkPhos Direct (GE Healthcare®). Chemiluminescent signals of increasing exposure periods were recorded on X-ray films. Non-saturated signals of individual Lk topoisomers and bins of pooled Lk distributions were quantified with ImageJ. The Lk mean of the Lk distributions was determined as previously described18 and illustrated in Supplementary Fig. 4.
Topo-seq
About 500 ng of yeast DNA, including that of minichromosomes hosting the nucleosome DNA library (about 0.5 ng), were loaded in two adjacent lanes of a gel containing 1.4% (w/v) agarose (Ultrapure grade, nzytech® MB05202) and electrophoresed at 2.5 V/cm for 18 h in TBE buffer. The gel slab of the first lane was kept at 4 °C in TBE, while the second lane was blot-transferred and probed to determine the position of the Lk mean of the overlapping Lk distributions. The gel slab of the first lane was then cut at the level of this Lk mean in two sections (A and B) of about 25 × 10 × 4 mm each, such that section A contained the top half of the overlapping Lk distributions; and section B, the bottom half. DNAs from both gel sections were eluted and captured using the Geneclean II kit®. DNA fragments of the nucleosome DNA library present in each section were amplified via PCR (NEB Taq Polymerase) by using as primers the sequences of the Asc1 and BamH1 adaptors described in Supplementary Fig. 1. The obtained amplicons of about 200 bp were sequenced in duplicate on two Flowcell Lane Index units (Illumina MiSeq v2 2×150 pb, paired-end reads) and the resulting FASTQ data subjected to QC using Cutadapt (1.12). The reads from two Flowcell lanes (sequencing replicates 1 and 2) were combined into a unique analysis dataset. The library sequences were then mapped to the Saccharomyces cerevisiae reference genome (R64-1-1 from Ensembl Genomes) using bowtie (v1.1.2). Once nucleosome coordinates were established, alignment and size statistics were calculated using SAMTools and Picard. Subsequent analyses were performed by integrating published nucleosome data sets13,48 and using BEDTools (v2.27) and Galaxy. The calculation of ∆Lk values using the Topo-seq DNA sequencing data is described in the results section and Supplementary Data 2. This process includes cunning the relative abundancies of nucleosomal DNA sequences in the gel sections A and B, the conversion of these partition probabilities into Z scores of a normal distribution, the transformation of Z scores into Lk values, and the adjustment of these ∆Lk values to take into account the length differences within the library of nucleosomal DNA sequences.
∆Lk modelling of nucleosomes
To model restrained ∆Lk values as a function of ∆Wr, the value of ∆Tw was fixed to +0.2 and restrained ∆Wr values were calculated from ∆Wr = N(1–sin ∂). The number of wrapped super-helical turns (N) was depicted as the length of the arcs in contact with the cylindrical histone core, leaving the entry and exit segments of DNA as detached tangents and with a pitch angle (∂) of 4o. As super-helical turns are left-handed, N < 0. The pitch angle (∂) of the super-helical turns was calculated from the height at the entry and exit points of 1.56 super-helical turns. To model the restrained ∆Lk as a function of ∆Tw, the nucleosome ∆Wr was fixed to −1.46 (= −1.56 (1–sin 4o) and restrained ∆Tw values of 0 and +0.4 were illustrated as the twisting angle observed at the entry and exit segments of DNA relative to ∆Tw in the cannon nucleosome (∆Tw = +0.2).
∆Lk correlation with DNA composition and nucleosome origin
Sequence properties of nucleosome DNAs including GC content, dinucleotide frequency and periodicity were analysed in R using the seqinR package. Mean dinucleotide frequencies were calculated for nucleosome ∆Lk values split into five quantiles. Dinucleotide periodicities were measured as the mean distance (in bps) of consecutive dinucleotides of the same type and calculated for nucleosome ∆Lk values split into ten quantiles. The native allocation of identified nucleosomes within a gene body, intergenic region and rDNA genes was defined by Jiang and Pugh (2009). Positional fuzziness of nucleosomes was determined as the standard deviation in bp of the nucleosome position from the 6 datasets compiled by Jian and Puhg13. Nucleosomes of telomeric regions were as defined in The Saccharomyces Genome Database (SGD)23. Statistic tests for the significance of ∆Lk correlations are described in the figure legends.
Statistics and reproducibility
Statistical tests were performed using Microsoft Excel version 16.7, GraphPad Prism version 8.0, and IBMSPSS Statistics version 19.0. The statistical test used for the data shown in each figure is noted in the corresponding figure legend, and significant statistical differences are noted as p-values. When indicated, values are reported as mean values ± standard deviation. No data were excluded from the analyses. The Topo-seq analysis was conducted once using the described library of nucleosomal DNAs. The reproducibility of the library results was validated by examining individual representative nucleosomes.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data generated in this study are available with this manuscript and its Supplementary Information. Source data have been deposited in NCBI’s Gene Expression Omnibus49 and are accessible through GEO Series accession number GSE228623. Source data are provided with this paper.
References
Kempfer, R. & Pombo, A. Methods for mapping 3D chromosome architecture. Nat. Rev. Genet 21, 207–226 (2020).
Jerkovic, I. & Cavalli, G. Understanding 3D genome organization by multidisciplinary methods. Nat. Rev. Mol. Cell Biol. 22, 511–528 (2021).
Roca, J. The torsional state of DNA within the chromosome. Chromosoma 120, 323–334 (2011).
Lavelle, C. Pack, unpack, bend, twist, pull, push: the physical side of gene expression. Curr. Opin. Genet Dev. 25, 74–84 (2014).
Jha, R. K., Levens, D. & Kouzine, F. Mechanical determinants of chromatin topology and gene expression. Nucleus 13, 94–115 (2022).
Luger, K., Mader, A. W., Richmond, R. K., Sargent, D. F. & Richmond, T. J. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature 389, 251–260 (1997).
Lai, W. K. M. & Pugh, B. F. Understanding nucleosome dynamics and their links to gene expression and DNA replication. Nat. Rev. Mol. Cell Biol. 18, 548–562 (2017).
Fierz, B. & Poirier, M. G. Biophysics of chromatin dynamics. Annu. Rev. Biophys. 48, 321–345 (2019).
Ahmad, K., Henikoff, S. & Ramachandran, S. Managing the steady state chromatin landscape by nucleosome dynamics. Annu Rev. Biochem 91, 183–195 (2022).
Zlatanova, J., Bishop, T. C., Victor, J. M., Jackson, V. & van Holde, K. The nucleosome family: dynamic and growing. Structure 17, 160–171 (2009).
Andrews, A. J. & Luger, K. Nucleosome structure(s) and stability: variations on a theme. Annu. Rev. Biophys. 40, 99–117 (2011).
Talbert, P. B. & Henikoff, S. The Yin and Yang of histone marks in transcription. Annu. Rev. Genomics Hum. Genet 22, 147–170 (2021).
Jiang, C. & Pugh, B. F. A compiled and systematic reference map of nucleosome positions across the Saccharomyces cerevisiae genome. Genome Biol. 10, R109 (2009).
Hsieh, T. H. et al. Mapping nucleosome resolution chromosome folding in yeast by Micro-C. Cell 162, 108–119 (2015).
Chereji, R. V., Ramachandran, S., Bryson, T. D. & Henikoff, S. Precise genome-wide mapping of single nucleosomes and linkers in vivo. Genome Biol. 19, 19 (2018).
Ohno, M. et al. Sub-nucleosomal genome structure reveals distinct nucleosome folding motifs. Cell 176, 520–534.e525 (2019).
Rossi, M. J. et al. A high-resolution protein architecture of the budding yeast genome. Nature 592, 309–314 (2021).
Segura, J. et al. Intracellular nucleosomes constrain a DNA linking number difference of −1.26 that reconciles the Lk paradox. Nat. Commun. 9, 3989 (2018).
Depew, D. E. & Wang, J. C. Conformational fluctuations of DNA helix. Proc. Natl Acad. Sci. USA 72, 4275–4279 (1975).
Hagerman, P. J. Flexibility of DNA. Annu. Rev. Biophys. Biophys. Chem. 17, 265–286 (1988).
Segal, E. & Widom, J. Poly(dA:dT) tracts: major determinants of nucleosome organization. Curr. Opin. Struct. Biol. 19, 65–71 (2009).
Geggier, S. & Vologodskii, A. Sequence dependence of DNA bending rigidity. Proc. Natl Acad. Sci. USA 107, 15421–15426 (2010).
Cherry, J. M. et al. Saccharomyces Genome Database: the genomics resource of budding yeast. Nucleic Acids Res. 40, D700–705 (2012).
Mirabella, A. & Gartenberg, M. R. Yeast telomeric sequences function as chromosomal anchorage points in vivo. EMBO J. 16, 523–533 (1997).
Mariezcurrena, A. & Uhlmann, F. Observation of DNA intertwining along authentic budding yeast chromosomes. Genes Dev. 31, 2151–2161 (2017).
Cheng, T. H., Li, Y. C. & Gartenberg, M. R. Persistence of an alternate chromatin structure at silenced loci in the absence of silencers. Proc. Natl Acad. Sci. USA 95, 5521–5526 (1998).
Vinogradov, A. E. DNA helix: the importance of being GC-rich. Nucleic Acids Res. 31, 1838–1844 (2003).
Hormeno, S. et al. Mechanical properties of high-G.C content DNA with a-type base-stacking. Biophys. J. 100, 1996–2005 (2011).
Basu, A. et al. Measuring DNA mechanics on the genome scale. Nature 589, 462–467 (2021).
Sheinin, M. Y., Li, M., Soltani, M., Luger, K. & Wang, M. D. Torque modulates nucleosome stability and facilitates H2A/H2B dimer loss. Nat. Commun. 4, 2579 (2013).
Kujirai, T. et al. Structural basis of the nucleosome transition during RNA polymerase II passage. Science 362, 595–598 (2018).
Dammann, R., Lucchini, R., Koller, T. & Sogo, J. M. Chromatin structures and transcription of rDNA in yeast Saccharomyces cerevisiae. Nucleic Acids Res 21, 2331–2338 (1993).
Merz, K. et al. Actively transcribed rRNA genes in S. cerevisiae are organized in a specialized chromatin associated with the high-mobility group protein Hmo1 and are largely devoid of histone molecules. Genes Dev. 22, 1190–1204 (2008).
Yuan, G. C. et al. Genome-scale identification of nucleosome positions in S. cerevisiae. Science 309, 626–630 (2005).
Oberbeckmann, E. et al. Absolute nucleosome occupancy map for the Saccharomyces cerevisiae genome. Genome Res. 29, 1996–2009 (2019).
Koerber, R. T., Rhee, H. S., Jiang, C. & Pugh, B. F. Interaction of transcriptional regulators with specific nucleosomes across the Saccharomyces genome. Mol. Cell 35, 889–902 (2009).
Krietenstein, N. et al. Genomic nucleosome organization reconstituted with pure proteins. Cell 167, 709–721.e712 (2016).
Kubik, S. et al. Sequence-directed action of RSC remodeler and general regulatory factors modulates +1 nucleosome position to facilitate transcription. Mol. Cell 71, 89–102.e105 (2018).
Rawal, Y. et al. SWI/SNF and RSC cooperate to reposition and evict promoter nucleosomes at highly expressed genes in yeast. Genes Dev. 32, 695–710 (2018).
Rhee, H. S. & Pugh, B. F. Genome-wide structure and organization of eukaryotic pre-initiation complexes. Nature 483, 295–301 (2012).
Wang, H., Schilbach, S., Ninov, M., Urlaub, H. & Cramer, P. Structures of transcription preinitiation complex engaged with the +1 nucleosome. Nat. Struct. Mol. Biol. 30, 226–232 (2023).
Bhardwaj, S. K. et al. Dinucleosome specificity and allosteric switch of the ISW1a ATP-dependent chromatin remodeler in transcription regulation. Nat. Commun. 11, 5913 (2020).
Adelman, K. & Lis, J. T. Promoter-proximal pausing of RNA polymerase II: emerging roles in metazoans. Nat. Rev. Genet. 13, 720–731 (2012).
Core, L. & Adelman, K. Promoter-proximal pausing of RNA polymerase II: a nexus of gene regulation. Genes Dev. 33, 960–982 (2019).
Hildreth, A. E. et al. The nucleosome DNA entry-exit site is important for transcription termination and prevention of pervasive transcription. Elife https://doi.org/10.7554/eLife.57757 (2020).
Shampay, J., Szostak, J. W. & Blackburn, E. H. DNA sequences of telomeres maintained in yeast. Nature 310, 154–157 (1984).
Soman, A. et al. Columnar structure of human telomeric chromatin. Nature 609, 1048–1055 (2022).
Ioshikhes, I. P., Albert, I., Zanton, S. J. & Pugh, B. F. Nucleosome positions predicted through comparative genomics. Nat. Genet. 38, 1210–1215 (2006).
Edgar, R., Domrachev, M. & Lash, A. E. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 30, 207–210 (2002).
Acknowledgements
The authors thank the CNAG Sequencing Service and the CRG Bioinformatics Core Facility for assistance; F. Azorín, G. Vicent and V. Zhurkin for discussing results. This work is supported by the Plan Estatal de Investigación Científica y Técnica of Spain, with grant PID2019-109482GB-I00 to J.R.; research fellowships BES-2012-061167 to J.S. and PRE2020-093378 to A.A-F.
Author information
Authors and Affiliations
Contributions
J.R. conceived Topo-seq and supervised the study. J.S. designed and performed the Topo-seq experiments. C.N. and J.S. designed and performed the bioinformatics analyses. O.D-I., B.M-G. and A.A-F. performed validation experiments. J.R. produced nucleosome models and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Andrey Krokhotin and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Segura, J., Díaz-Ingelmo, O., Martínez-García, B. et al. Nucleosomal DNA has topological memory. Nat Commun 15, 4526 (2024). https://doi.org/10.1038/s41467-024-49023-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-49023-4
- Springer Nature Limited