Abstract
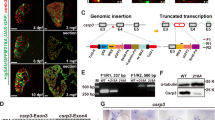
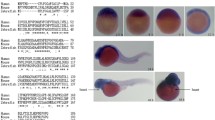
Ca2+ signaling is critical for heart development; however, the precise roles and regulatory pathways of Ca2+ transport proteins in cardiogenesis remain largely unknown. Sodium-calcium exchanger 1 (Ncx1) is responsible for Ca2+ efflux in cardiomyocytes. It is involved in cardiogenesis, while the mechanism is unclear. Here, using the forward genetic screening in zebrafish, we identified a novel mutation at a highly-conserved leucine residue in ncx1 gene (mutantLDD353/ncx1hL154P) that led to smaller hearts with reduced heart rate and weak contraction. Mechanistically, the number of ventricular but not atrial cardiomyocytes was reduced in ncx1hL154P zebrafish. These defects were mimicked by knockdown or knockout of ncx1h. Moreover, ncx1hL154P had cytosolic and mitochondrial Ca2+ overloading and Ca2+ transient suppression in cardiomyocytes. Furthermore, ncx1hL154P and ncx1h morphants downregulated cardiac transcription factors hand2 and gata4 in the cardiac regions, while overexpression of hand2 and gata4 partially rescued cardiac defects including the number of ventricular myocytes. These findings demonstrate an essential role of the novel 154th leucine residue in the maintenance of Ncx1 function in zebrafish, and reveal previous unrecognized critical roles of the 154th leucine residue and Ncx1 in the formation of ventricular cardiomyocytes by at least partially regulating the expression levels of gata4 and hand2.
Similar content being viewed by others
References
Barth, E., Stammler, G., Speiser, B., and Schaper, J. (1992). Ultrastructural quantitation of mitochondria and myofilaments in cardiac muscle from 10 different animal species including man. J Mol Cell Cardiol 24, 669–681.
Berridge, M.J. (2016). The inositol trisphosphate/calcium signaling pathway in health and disease. Physiol Rev 96, 1261–1296.
Brini, M., and Carafoli, E. (2009). Calcium pumps in health and disease. Physiol Rev 89, 1341–1378.
Bruneau, B.G. (2008). The developmental genetics of congenital heart disease. Nature 451, 943–948.
Chen, J.N., Haffter, P., Odenthal, J., Vogelsang, E., Brand, M., van Eeden, F.J., Furutani-Seiki, M., Granato, M., Hammerschmidt, M., Heisenberg, C.P., et al. (1996). Mutations affecting the cardiovascular system and other internal organs in zebrafish. Development 123, 293–302.
Dai, Y.S., Cserjesi, P., Markham, B.E., and Molkentin, J.D. (2002). The transcription factors GATA4 and dHAND physically interact to synergistically activate cardiac gene expression through a p300-dependent mechanism. J Biol Chem 277, 24390–24398.
Deacon, D.C., Nevis, K.R., Cashman, T.J., Zhou, Y., Zhao, L., Washko, D., Guner-Ataman, B., Burns, C.G., and Burns, C.E. (2010). The miR-143-adducin3 pathway is essential for cardiac chamber morphogenesis. Development 137, 1887–1896.
Ebert, A.M., Hume, G.L., Warren, K.S., Cook, N.P., Burns, C.G., Mohideen, M.A., Siegal, G., Yelon, D., Fishman, M.C., and Garrity, D.M. (2005). Calcium extrusion is critical for cardiac morphogenesis and rhythm in embryonic zebrafish hearts. Proc Natl Acad Sci USA 102, 17705–17710.
Fu, J., Yu, H., Wang, R., Liang, J., and Yang, H. (2006). Developmental regulation of intracellular calcium transients during cardiomyocyte differentiation of mouse embryonic stem cells1. Acta Pharmacol Sin 27, 901–910.
Gao, L., Li, D., Ma, K., Zhang, W., Xu, T., Fu, C., Jing, C., Jia, X., Wu, S., Sun, X., et al. (2015). TopBP1 governs hematopoietic stem/progenitor cells survival in zebrafish definitive hematopoiesis. PLoS Genet 11, e1005346.
Graier, W.F., Frieden, M., and Malli, R. (2007). Mitochondria and Ca2+ signaling: old guests, new functions. Pflugers Arch Eur J Physiol 455, 375–396.
Guo, A., and Yang, H.T. (2009). Ca2+ removal mechanisms in mouse embryonic stem cell-derived cardiomyocytes. Am J Physiol Cell Physiol 297, C732–C741.
Hinits, Y., Pan, L., Walker, C., Dowd, J., Moens, C.B., and Hughes, S.M. (2012). Zebrafish Mef2ca and Mef2cb are essential for both first and second heart field cardiomyocyte differentiation. Dev Biol 369, 199–210.
Huang, J., Zhang, M., Zhang, P., Liang, H., Ouyang, K., and Yang, H.T. (2016). Coupling switch of P2Y-IP3 receptors mediates differential Ca2+ signaling in human embryonic stem cells and derived cardiovascular progenitor cells. Purinergic Signal 12, 465–478.
Jin, D., Ni, T.T., Hou, J., Rellinger, E., and Zhong, T.P. (2009). Promoter analysis of ventricular myosin heavy chain (vmhc) in zebrafish embryos. Dev Dyn 238, 1760–1767.
Jing, C.B., Chen, Y., Dong, M., Peng, X.L., Jia, X.E., Gao, L., Ma, K., Deng, M., Liu, T.X., Zon, L.I., et al. (2013). Phospholipase C gamma-1 is required for granulocyte maturation in zebrafish. Dev Biol 374, 24–31.
Khananshvili, D. (2013). The SLC8 gene family of sodium-calcium exchangers (NCX)—Structure, function, and regulation in health and disease. Mol Aspects Med 34, 220–235.
Koban, M.U., Brugh, S.A., Riordon, D.R., Dellow, K.A., Yang, H.T., Tweedie, D., and Boheler, K.R. (2001). A distant upstream region of the rat multipartite Na+-Ca2+ exchanger NCX1 gene promoter is sufficient to confer cardiac-specific expression. Mech Dev 109, 267–279.
Koushik, S.V., Bundy, J., and Conway, S.J. (1999). Sodium-calcium exchanger is initially expressed in a heart-restricted pattern within the early mouse embryo. Mech Dev 88, 119–122.
Koushik, S.V., Wang, J., Rogers, R., Moskophidis, D., Lambert, N.A., Creazzo, T.L., and Conway, S.J. (2001). Targeted inactivation of the sodium-calcium exchanger (Ncx1) results in the lack of a heartbeat and abnormal myofibrillar organization. FASEB J 15, 1209–1211.
Langenbacher, A.D., Dong, Y., Shu, X., Choi, J., Nicoll, D.A., Goldhaber, J.I., Philipson, K.D., and Chen, J.N. (2005). Mutation in sodium-calcium exchanger 1 (NCX1) causes cardiac fibrillation in zebrafish. Proc Natl Acad Sci USA 102, 17699–17704.
Laude, A.J., and Simpson, A.W.M. (2009). Compartmentalized signalling: Ca2+ compartments, microdomains and the many facets of Ca2+ signalling. FEBS J 276, 1800–1816.
Liang, J., Wang, Y.J., Tang, Y., Cao, N., Wang, J., and Yang, H.T. (2010). Type 3 inositol 1,4,5-trisphosphate receptor negatively regulates apoptosis during mouse embryonic stem cell differentiation. Cell Death Differ 17, 1141–1154.
Linask, K.L., and Linask, K.K. (2010). Calcium channel blockade in embryonic cardiac progenitor cells disrupts normal cardiac cell differentiation. Stem Cells Dev 19, 1959–1965.
Liu, D., Wang, Z., Xiao, A., Zhang, Y., Li, W., Zu, Y., Yao, S., Lin, S., and Zhang, B. (2014). Efficient gene targeting in zebrafish mediated by a zebrafish-codon-optimized cas9 and evaluation of off-targeting effect. J Genet Genomics 41, 43–46.
Lu, F., Langenbacher, A.D., and Chen, J.N. (2016). Transcriptional regulation of heart development in zebrafish. J Cardiovasc Dev Dis 3.
McFadden, D.G., Barbosa, A.C., Richardson, J.A., Schneider, M.D., Srivastava, D., and Olson, E.N. (2005). The Hand1 and Hand2 transcription factors regulate expansion of the embryonic cardiac ventricles in a gene dosage-dependent manner. Development 132, 189–201.
Meng, Z.Z., Liu, W., Xia, Y., Yin, H.M., Zhang, C.Y., Su, D., Yan, L.F., Gu, A.H., and Zhou, Y. (2017). The pro-inflammatory signalling regulator Stat4 promotes vasculogenesis of great vessels derived from endothelial precursors. Nat Commun 8, 14640.
Mizuno, H., Sassa, T., Higashijima, S.I., Okamoto, H., and Miyawaki, A. (2013). Transgenic zebrafish for ratiometric imaging of cytosolic and mitochondrial Ca2+ response in teleost embryo. Cell Calcium 54, 236–245.
Moorman, A.F.M., and Christoffels, V.M. (2003). Cardiac chamber formation: development, genes, and evolution. Physiol Rev 83, 1223–1267.
North, T.E., Goessling, W., Peeters, M., Li, P., Ceol, C., Lord, A.M., Weber, G.J., Harris, J., Cutting, C.C., Huang, P., et al. (2009). Hematopoietic stem cell development is dependent on blood flow. Cell 137, 736–748.
Ottolia, M., Nicoll, D.A., and Philipson, K.D. (2005). Mutational analysis of the α-1 repeat of the cardiac Na+-Ca2+ exchanger. J Biol Chem 280, 1061–1069.
Porter Jr., G.A., Makuck, R.F., and Rivkees, S.A. (2003). Intracellular calcium plays an essential role in cardiac development. Dev Dyn 227, 280–290.
Prall, O.W.J., Menon, M.K., Solloway, M.J., Watanabe, Y., Zaffran, S., Bajolle, F., Biben, C., McBride, J.J., Robertson, B.R., Chaulet, H., et al. (2007). An Nkx2–5/Bmp2/Smad1 negative feedback loop controls heart progenitor specification and proliferation. Cell 128, 947–959.
Prendiville, T., Jay, P.Y., and Pu, W.T. (2014). Insights into the genetic structure of congenital heart disease from human and murine studies on monogenic disorders. Cold Spring Harb Perspect Med 4, a013946.
Pucéat, M., and Jaconi, M. (2005). Ca2+ signalling in cardiogenesis. Cell Calcium 38, 383–389.
Schindelin, J., Arganda-Carreras, I., Frise, E., Kaynig, V., Longair, M., Pietzsch, T., Preibisch, S., Rueden, C., Saalfeld, S., Schmid, B., et al. (2012). Fiji: an open-source platform for biological-image analysis. Nat Methods 9, 676–682.
Schindler, Y.L., Garske, K.M., Wang, J., Firulli, B.A., Firulli, A.B., Poss, K.D., and Yelon, D. (2014). Hand2 elevates cardiomyocyte production during zebrafish heart development and regeneration. Development 141, 3112–3122.
Schoenebeck, J.J., Keegan, B.R., and Yelon, D. (2007). Vessel and blood specification override cardiac potential in anterior mesoderm. Dev Cell 13, 254–267.
Sehnert, A.J., Huq, A., Weinstein, B.M., Walker, C., Fishman, M., and Stainier, D.Y.R. (2002). Cardiac troponin T is essential in sarcomere assembly and cardiac contractility. Nat Genet 31, 106–110.
Song, Q., Huang, M., Wang, B., Kang, X., and Wang, C. (2018). Bidirectional regulation of Ca2+ in exo-endocytosis coupling. Sci China Life Sci 61, 1583–1585.
Srivastava, D., Thomas, T., Lin, Q., Kirby, M.L., Brown, D., and Olson, E. N. (1997). Regulation of cardiac mesodermal and neural crest development by the bHLH transcription factor, dHAND. Nat Genet 16, 154–160.
Stainier, D.Y., Fouquet, B., Chen, J.N., Warren, K.S., Weinstein, B.M., Meiler, S.E., Mohideen, M.A., Neuhauss, S.C., Solnica-Krezel, L., Schier, A.F., et al. (1996). Mutations affecting the formation and function of the cardiovascular system in the zebrafish embryo. Development 123, 285–292.
Staudt, D., and Stainier, D. (2012). Uncovering the molecular and cellular mechanisms ofheart development using the zebrafish. Annu Rev Genet 46, 397–418.
Sun, Y.M., Wang, J., Qiu, X.B., Yuan, F., Li, R.G., Xu, Y.J., Qu, X.K., Shi, H.Y., Hou, X.M., Huang, R.T., et al. (2016). A HAND2 loss-of-function mutation causes familial ventricular septal defect and pulmonary stenosis. G3 6, 987–992.
Tong, X., Zu, Y., Li, Z., Li, W., Ying, L., Yang, J., Wang, X., He, S., Liu, D., Zhu, Z., et al. (2014). Kctd10 regulates heart morphogenesis by repressing the transcriptional activity of Tbx5a in zebrafish. Nat Commun 5, 3153.
Tse, M.K., Hung, T.S., Chan, C.M., Wong, T., Dorothea, M., Leclerc, C., Moreau, M., Miller, A.L., and Webb, S.E. (2018). Identification of Ca2+ signaling components in neural stem/progenitor cells during differentiation into neurons and glia in intact and dissociated zebrafish neurospheres. Sci China Life Sci 61, 1352–1368.
Tu, S., and Chi, N.C. (2012). Zebrafish models in cardiac development and congenital heart birth defects. Differentiation 84, 4–16.
Vincentz, J.W., Barnes, R.M., and Firulli, A.B. (2011). Hand factors as regulators of cardiac morphogenesis and implications for congenital heart defects. Birth Defects Res A Clin Mol Teratol 91, 485–494.
Wakimoto, K., Kobayashi, K., Kuro-O, M., Yao, A., Iwamoto, T., Yanaka, N., Kita, S., Nishida, A., Azuma, S., Toyoda, Y., et al. (2000). Targeted disruption of Na+/Ca2+ exchanger gene leads to cardiomyocyte apoptosis and defects in heartbeat. J Biol Chem 275, 36991–36998.
Wang, Y.J., Huang, J., Liu, W., Kou, X., Tang, H., Wang, H., Yu, X., Gao, S., Ouyang, K., and Yang, H.T. (2017). IP3R-mediated Ca2+ signals govern hematopoietic and cardiac divergence of Flk1+ cells via the calcineurin-NFATc3-Etv2 pathway. J Mol Cell Biol 9, 274–288.
Wansleeben, C., Feitsma, H., Tertoolen, L., Kroon, C., Guryev, V., Cuppen, E., and Meijlink, F. (2010). A novel mutant allele of Ncx1: a single amino acid substitution leads to cardiac dysfunction. Int J Dev Biol 54, 1465–1470.
Yamagishi, H., Yamagishi, C., Nakagawa, O., Harvey, R.P., Olson, E.N., and Srivastava, D. (2001). The combinatorial activities of Nkx2.5 and dHAND are essential for cardiac ventricle formation. Dev Biol 239, 190–203.
Yelon, D., Horne, S.A., and Stainier, D.Y.R. (1999). Restricted expression of cardiac myosin genes reveals regulated aspects of heart tube assembly in zebrafish. Dev Biol 214, 23–37.
Zeisberg, E.M., Ma, Q., Juraszek, A.L., Moses, K., Schwartz, R.J., Izumo, S., and Pu, W.T. (2005). Morphogenesis of the right ventricle requires myocardial expression of Gata4. J Clin Invest 115, 1522–1531.
Zhou, Y., Cashman, T.J., Nevis, K.R., Obregon, P., Carney, S.A., Liu, Y., Gu, A., Mosimann, C., Sondalle, S., Peterson, R.E., et al. (2011). Latent TGF-β binding protein 3 identifies a second heart field in zebrafish. Nature 474, 645–648.
Zhou, Y., and Zon, L.I. (2011). The zon laboratory guide to positional cloning in zebrafish. Methods Cell Biol 104, 287–309.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (81520108004, 81470422), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA16010201), and National Key R&D Program of China (2017YFA 0103700, 2016YFC1301204) to H.-T.Y., and Shanghai Natural Science Foundation (17ZR1435500) to J.H. We acknowledge Dr. Atsushi Miyawaki and Dr. Akiko Ishioka for providing the two Ca2+ indicator transgenic zebrafish lines, and Prof. Tao Zhong for providing the vmhc-EGFP plasmid. We also thank Min Den, Yi Jin, Mei Dong, and Yukun Jiang for technical assistance.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no conflict of interest. All applicable institutional and national guidelines for the care and use of animals were followed.
Supplementary Materials
Supplementary material, approximately 5.37 MB.
Supplementary material, approximately 4.97 MB.
Supplementary material, approximately 4.87 MB.
11427_2019_1706_MOESM4_ESM.docx
Cardiac Na+-Ca2+ exchanger 1 (ncx1h) is critical for the ventricular cardiomyocyte formation via regulating the expression levels of gata4 and hand2 in zebrafish
Rights and permissions
About this article
Cite this article
Chu, L., Yin, H., Gao, L. et al. Cardiac Na+-Ca2+ exchanger 1 (ncx1h) is critical for the ventricular cardiomyocyte formation via regulating the expression levels of gata4 and hand2 in zebrafish. Sci. China Life Sci. 64, 255–268 (2021). https://doi.org/10.1007/s11427-019-1706-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11427-019-1706-1