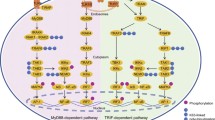
Abstract
Toll-like receptors sense invading pathogens by recognizing a wide variety of conserved pathogen-associated molecular patterns (PAMPs). The members of TLR family selectively utilize adaptor proteins MyD88, TRIF, TIRAP and TRAM to activate overlapping but distinct signal transduction pathways which trigger production of different panels of mediators such as proinflammatory cytokines and type I interferon. These mediators not only control innate immunity but also direct subsequently developed adaptive immunity. TLR activation is strictly and finely regulated at multiple levels of the signal transduction pathways.
Similar content being viewed by others
References
Lemaitre B, Nicolas E, Michaut L, et al. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell, 1996, 86: 973–983, 10.1016/S0092-8674(00)80172-5, 8808632, 1:CAS:528:DyaK28XlvV2nsLY%3D
Medzhitov R, Preston-Hurlburt P, Janeway C A, et al. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature, 1997, 388: 394–397, 10.1038/41131, 9237759, 1:CAS:528:DyaK2sXkvVChurc%3D
Akira S. Toll-like receptor signaling. J Biol Chem, 2003, 278: 38105–38108, 10.1074/jbc.R300028200, 12893815, 1:CAS:528:DC%2BD3sXnslSqtr8%3D
Schnare M, Barton G M, Holt A C, et al. Toll-like receptors control activation of adaptive immune responses. Nat Immunol, 2001, 2: 947–950, 10.1038/ni712, 11547333, 1:CAS:528:DC%2BD3MXnsVCis74%3D
Liew F Y, Xu D, Brint E K, et al. Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol, 2005, 5: 446–458, 10.1038/nri1630, 15928677, 1:CAS:528:DC%2BD2MXks1GmsLo%3D
Ozinsky A, Underhill D M, Fontenot J D, et al. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci USA, 2000, 97: 13766–13771, 10.1073/pnas.250476497, 11095740, 1:CAS:528:DC%2BD3cXoslyns7w%3D
Takeuchi O, Sato S, Horiuchi T, et al. Cutting edge: Role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J Immunol 2002, 169: 10–14, 12077222, 1:CAS:528:DC%2BD38XltVKjtbs%3D
Omueti K O, Beyer J M, Johnson C M, et al. Domain exchange between human toll-like receptors 1 and 6 reveals a region required for lipopeptide discrimination. J Biol Chem, 2005, 280: 36616–36625, 10.1074/jbc.M504320200, 16129684, 1:CAS:528:DC%2BD2MXhtFKht7fO
Liu L, Botos I, Wang Y, et al. Structural basis of toll-like receptor 3 signaling with double-stranded RNA. Science, 2008, 320: 379–381, 10.1126/science.1155406, 18420935, 1:CAS:528:DC%2BD1cXks1GhsLk%3D
Park B S, Song D H, Kim H M, et al. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature, 2009, 458: 1191–1195, 10.1038/nature07830, 19252480, 1:CAS:528:DC%2BD1MXisVOrtLk%3D
O’Neill L A, Bowie A G. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol, 2007, 7: 353–364, 10.1038/nri2079, 17457343, 1:CAS:528:DC%2BD2sXksFeks7g%3D
LeBouder E, Rey-Nores J E, Rushmere N K, et al. Soluble forms of Toll-like receptor (TLR)2 capable of modulating TLR2 signaling are present in human plasma and breast milk. J Immunol, 2003, 171: 6680–6689, 14662871, 1:CAS:528:DC%2BD3sXpsV2rt7Y%3D
Iwami K I, Matsuguchi T, Masuda A, et al. Cutting edge: Naturally occurring soluble form of mouse Toll-like receptor 4 inhibits lipopolysaccharide signaling. J Immunol, 2000, 165: 6682–6686, 11120784, 1:CAS:528:DC%2BD3cXovFyhtL4%3D
Kagan JC, Su T, Horng T, et al. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol, 2008, 9: 361–368, 10.1038/ni1569, 18297073, 1:CAS:528:DC%2BD1cXjtlGms7w%3D
Cao X. Forefront of progress in immunology. Beijing: People’s Medical Publishing House, 2009. 136–160
Lord K A, Hoffman-Liebermann B, Liebermann D A. Nucleotide sequence and expression of a cDNA encoding MyD88, a novel myeloid differentiation primary response gene induced by IL6. Oncogene, 1990, 5: 1095–1097, 2374694, 1:CAS:528:DyaK3cXmt1GnsbY%3D
Muzio M, Ni J, Feng P, et al. IRAK (Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL-1 signaling. Science 1997, 278: 1612–1615, 10.1126/science.278.5343.1612, 9374458, 1:CAS:528:DyaK2sXnslCisbc%3D
Wesche H, Henzel W J, Shillinglaw W, et al. MyD88: An adapter that recruits IRAK to the IL-1 receptor complex. Immunity, 1997, 7: 837–847, 10.1016/S1074-7613(00)80402-1, 9430229, 1:CAS:528:DyaK1cXjsFahtg%3D%3D
Kawai T, Adachi O, Ogawa T, et al. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity, 1999, 11: 115–122, 10.1016/S1074-7613(00)80086-2, 10435584, 1:CAS:528:DyaK1MXltFCgt7w%3D
Janssens S, Burns K, Tschopp J, et al. Regulation of interleukin-1- and lipopolysaccharide-induced NF-kappaB activation by alternative splicing of MyD88. Curr Biol, 2002, 12: 467–471, 10.1016/S0960-9822(02)00712-1, 11909531, 1:CAS:528:DC%2BD38XitlGgur8%3D
Burns K, Janssens S, Brissoni B, et al. Inhibition of interleukin 1 receptor/Toll-like receptor signaling through the alternatively spliced, short form of MyD88 is due to its failure to recruit IRAK-4. J Exp Med, 2003, 197: 263–268, 10.1084/jem.20021790, 12538665
Fitzgerald K A, Palsson-McDermott E M, Bowie A G, et al. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature, 2001, 413: 78–83, 10.1038/35092578, 11544529, 1:CAS:528:DC%2BD3MXmvFSrsrg%3D
Yamamoto M, Sato S, Hemmi H, et al. Essential role for TIRAP in activation of the signalling cascade shared by TLR2 and TLR4. Nature, 2002, 420: 324–329, 10.1038/nature01182, 12447441, 1:CAS:528:DC%2BD38XovVektL4%3D
Horng T, Barton G M, Flavell R A, et al. The adaptor molecule TIRAP provides signalling specificity for Toll-like receptors. Nature, 2002, 420: 329–333, 10.1038/nature01180, 12447442, 1:CAS:528:DC%2BD38XovVekt7c%3D
Kagan J C, Medzhitov R. Phosphoinositide-mediated adaptor recruitment controls Toll-like receptor signaling. Cell, 2006, 125: 943–955, 10.1016/j.cell.2006.03.047, 16751103, 1:CAS:528:DC%2BD28Xls1Shtro%3D
Ohnishi H, Tochio H, Kato Z, et al. Structural basis for the multiple interactions of the MyD88 TIR domain in TLR4 signaling. Proc Natl Acad Sci USA, 2009, 106: 10260–10265, 10.1073/pnas.0812956106, 19506249
Kanakaraj P, Schafer P H, Cavender D E, et al. Interleukin (IL)-1 receptor-associated kinase (IRAK) requirement for optimal induction of multiple IL-1 signaling pathways and IL-6 production. J Exp Med, 1998, 187: 2073–2079, 10.1084/jem.187.12.2073, 9625767, 1:CAS:528:DyaK1cXktVeit7o%3D
Thomas J A, Allen J L, Tsen M, et al. Impaired cytokine signaling in mice lacking the IL-1 receptor-associated kinase. J Immunol, 1999, 163: 978–984, 10395695, 1:CAS:528:DyaK1MXksVensrc%3D
Li S, Strelow A, Fontana E J, et al. IRAK-4: A novel member of the IRAK family with the properties of an IRAK-kinase. Proc Natl Acad Sci USA, 2002, 99: 5567–5572, 10.1073/pnas.082100399, 11960013, 1:CAS:528:DC%2BD38XjtFKlt7s%3D
Kobayashi K, Hernandez L D, Galan J E, et al. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell, 2002, 110: 191–202, 10.1016/S0092-8674(02)00827-9, 12150927, 1:CAS:528:DC%2BD38XlvV2htrw%3D
Picard C, Puel A, Bonnet M, et al. Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science, 2003, 299: 2076–2079, 10.1126/science.1081902, 12637671, 1:CAS:528:DC%2BD3sXitlCisLo%3D
Kim T W, Staschke K, Bulek K, et al. A critical role for IRAK4 kinase activity in Toll-like receptor-mediated innate immunity. J Exp Med, 2007, 204: 1025–1036, 10.1084/jem.20061825, 17470642, 1:CAS:528:DC%2BD2sXlsFSjsbc%3D
Kawagoe T, Sato S, Matsushita K, et al. Sequential control of Toll-like receptor-dependent responses by IRAK1 and IRAK2. Nat Immunol, 2008, 9: 684–691, 10.1038/ni.1606, 18438411, 1:CAS:528:DC%2BD1cXmtVWqur0%3D
Ye H, Arron J R, Lamothe B, et al. Distinct molecular mechanism for initiating TRAF6 signalling. Nature, 2002, 418:443–447, 10.1038/nature00888, 12140561, 1:CAS:528:DC%2BD38XlsFajsbc%3D
Jacinto R, Hartung T, McCall C, et al. Lipopolysaccharide- and lipoteichoic acid-induced tolerance and cross-tolerance: Distinct alterations in IL-1 receptor-associated kinase. J Immunol, 2002, 168: 6136–6141, 12055225, 1:CAS:528:DC%2BD38XksFWmt70%3D
Li X, Commane M, Burns C, et al. Mutant cells that do not respond to interleukin-1 (IL-1) reveal a novel role for IL-1 receptor-associated kinase. Mol Cell Biol, 1999, 19: 4643–4652, 10373513, 1:CAS:528:DyaK1MXktVCgs7c%3D
Jensen L E, Whitehead A S. IRAK1b, a novel alternative splice variant of interleukin-1 receptor-associated kinase (IRAK), mediates interleukin-1 signaling and has prolonged stability. J Biol Chem, 2001, 276: 29037–29044, 10.1074/jbc.M103815200, 11397809, 1:CAS:528:DC%2BD3MXlvFSqsL8%3D
Rao N, Nguyen S, Ngo K, et al. A novel splice variant of interleukin-1 receptor (IL-1R)-associated kinase 1 plays a negative regulatory role in Toll/IL-1R-induced inflammatory signaling. Mol Cell Biol, 2005, 25: 6521–6532, 10.1128/MCB.25.15.6521-6532.2005, 16024789, 1:CAS:528:DC%2BD2MXntVShurc%3D
Hardy M P, O’Neill L A. The murine IRAK2 gene encodes four alternatively spliced isoforms, two of which are inhibitory. J Biol Chem, 2004, 279: 27699–27708, 10.1074/jbc.M403068200, 15082713, 1:CAS:528:DC%2BD2cXkvFGnurY%3D
Wang C, Deng L, Hong M, et al. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature, 2001, 412: 346–351, 10.1038/35085597, 11460167, 1:CAS:528:DC%2BD3MXlsFGjsLs%3D
Kawai T, Sato S, Ishii K J, et al. Interferon-alpha induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat Immunol, 2004, 5: 1061–1068, 10.1038/ni1118, 15361868, 1:CAS:528:DC%2BD2cXnvFCgs7w%3D
Hacker H, Redecke V, Blagoev B, et al. Specificity in Toll-like receptor signalling through distinct effector functions of TRAF3 and TRAF6. Nature, 2006, 439: 204–207, 10.1038/nature04369, 16306937, 1:CAS:528:DC%2BD28XislKhtA%3D%3D
Oganesyan G, Saha S K, Guo B, et al. Critical role of TRAF3 in the Toll-like receptor-dependent and -independent antiviral response. Nature, 2006, 439: 208–211, 10.1038/nature04374, 16306936, 1:CAS:528:DC%2BD28XislKhtQ%3D%3D
Zarnegar B J, Wang Y, Mahoney D J, et al. Noncanonical NF-kappaB activation requires coordinated assembly of a regulatory complex of the adaptors cIAP1, cIAP2, TRAF2 and TRAF3 and the kinase NIK. Nat Immunol, 2008, 9: 1371–1378, 10.1038/ni.1676, 18997794, 1:CAS:528:DC%2BD1cXhtlOjsbrM
Zarnegar B, Yamazaki S, He J Q, et al. Control of canonical NF-kappaB activation through the NIK-IKK complex pathway. Proc Natl Acad Sci USA, 2008, 105: 3503–3508, 10.1073/pnas.0707959105, 18292232
Sato S, Sanjo H, Takeda K, et al. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol, 2005, 6: 1087–1095, 10.1038/ni1255, 16186825, 1:CAS:528:DC%2BD2MXhtFamsb7K
Takaesu G, Kishida S, Hiyama A, et al. TAB2, a novel adaptor protein, mediates activation of TAK1 MAPKKK by linking TAK1 to TRAF6 in the IL-1 signal transduction pathway. Mol Cell, 2000, 5: 649–658, 10.1016/S1097-2765(00)80244-0, 10882101, 1:CAS:528:DC%2BD3cXjtFSqt7w%3D
Xia Z P, Sun L, Chen X, et al. Direct activation of protein kinases by unanchored polyubiquitin chains. Nature, 2009, 7620: 114–119, 10.1038/nature08247, 1:CAS:528:DC%2BD1MXpsleru70%3D
Liu X, Yao M, Li N, et al. CaMKII promotes TLR-triggered proinflammatory cytokine and type I interferon production by directly binding and activating TAK1 and IRF3 in macrophages. Blood, 2008, 112: 4961–4970, 10.1182/blood-2008-03-144022, 18818394, 1:CAS:528:DC%2BD1cXhsFWms73N
Yamamoto M, Sato S, Mori K, et al. Cutting edge: A novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-beta promoter in the Toll-like receptor signaling. J Immunol, 2002, 169: 6668–6672, 12471095, 1:CAS:528:DC%2BD38XpsFCqu74%3D
Hoebe K, Du X, Georgel P, et al. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature, 2003, 424: 743–748, 10.1038/nature01889, 12872135, 1:CAS:528:DC%2BD3sXmt1ant7w%3D
Yamamoto M, Sato S, Hemmi H, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science, 2003, 301: 640–643, 10.1126/science.1087262, 12855817, 1:CAS:528:DC%2BD3sXlvVKnsLs%3D
Oshiumi H, Matsumoto M, Funami K, et al. TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-beta induction. Nat Immunol, 2003, 4: 161–167, 10.1038/ni886, 12539043, 1:CAS:528:DC%2BD3sXmslKisA%3D%3D
Sato S, Sugiyama M, Yamamoto M, et al. Toll/IL-1 receptor domain-containing adaptor inducing IFN-beta (TRIF) associates with TNF receptor-associated factor 6 and TANK-binding kinase 1, and activates two distinct transcription factors, NF-kappa B and IFN-regulatory factor-3, in the Toll-like receptor signaling. J Immunol, 2003, 171: 4304–4310, 14530355, 1:CAS:528:DC%2BD3sXnvVOkt7Y%3D
Jiang Z, Mak T W, Sen G, et al. Toll-like receptor 3-mediated activation of NF-kappaB and IRF3 diverges at Toll-IL-1 receptor domain-containing adapter inducing IFN-beta. Proc Natl Acad Sci USA, 2004, 101: 3533–3538, 10.1073/pnas.0308496101, 14982987, 1:CAS:528:DC%2BD2cXisFWmtrw%3D
An H, Hou J, Zhou J, et al. Phosphatase SHP-1 promotes TLR- and RIG-I-activated production of type I interferon by inhibiting the kinase IRAK1. Nat Immunol, 2008, 9: 542–550, 10.1038/ni.1604, 18391954, 1:CAS:528:DC%2BD1cXkvVOqtbo%3D
Wietek C, Miggin S M, Jefferies C A, et al. Interferon regulatory factor-3-mediated activation of the interferon-sensitive response element by Toll-like receptor (TLR) 4 but not TLR3 requires the p65 subunit of NF-kappa. J Biol Chem, 2003, 278: 50923–50931, 10.1074/jbc.M308135200, 14557267, 1:CAS:528:DC%2BD3sXpslOhsr0%3D
Palsson-McDermott E M, Doyle S L, McGettrick A F, et al. TAG, a splice variant of the adaptor TRAM, negatively regulates the adaptor MyD88-independent TLR4 pathway. Nat Immunol, 2009, 10: 579–586, 10.1038/ni.1727, 19412184, 1:CAS:528:DC%2BD1MXltlSrsbs%3D
Brint E K, Xu D, Liu H, et al. ST2 is an inhibitor of interleukin 1 receptor and Toll-like receptor 4 signaling and maintains endotoxin tolerance. Nat Immunol, 2004, 5: 373–379, 10.1038/ni1050, 15004556, 1:CAS:528:DC%2BD2cXisF2ktb8%3D
Sweet M J, Leung B P, Kang D, et al. A novel pathway regulating lipopolysaccharide-induced shock by ST2/T1 via inhibition of Toll-like receptor 4 expression. J Immunol, 2001, 166: 6633–6639, 11359817, 1:CAS:528:DC%2BD3MXjvFartbk%3D
Wald D, Qin J, Zhao Z, et al. SIGIR R, a negative regulator of Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol, 2003, 4: 920–927, 10.1038/ni968, 12925853, 1:CAS:528:DC%2BD3sXms12gtLw%3D
Divanovic S, Trompette A, Atabani S F, et al. Negative regulation of Toll-like receptor 4 signaling by the Toll-like receptor homolog RP105. Nat Immunol, 2005, 6: 571–578, 10.1038/ni1198, 15852007, 1:CAS:528:DC%2BD2MXktlWitrg%3D
Diehl G E, Yue H H, Hsieh K, et al. TRAIL-R as a negative regulator of innate immune cell responses. Immunity, 2004, 21: 877–889, 10.1016/j.immuni.2004.11.008, 15589175, 1:CAS:528:DC%2BD2MXjvFGjuw%3D%3D
Rui Y, Liu X, Li N, et al. PECAM-1 ligation negatively regulates TLR4 signaling in macrophages. J Immunol, 2007, 179: 7344–7351, 18025177, 1:CAS:528:DC%2BD2sXhtlajt7%2FE
Hamerman J A, Tchao N K, Lowell C A, et al. Enhanced Toll-like receptor responses in the absence of signaling adaptor DAP12. Nat Immunol, 2005, 6: 579–586, 10.1038/ni1204, 15895090, 1:CAS:528:DC%2BD2MXktlWit7w%3D
Turnbull I R, McDunn J E, Takai T, et al. DAP12 (KARAP) amplifies inflammation and increases mortality from endotoxemia and septic peritonitis. J Exp Med, 2005, 202: 363–369, 10.1084/jem.20050986, 16061725, 1:CAS:528:DC%2BD2MXntFCjt7s%3D
Carty M, Goodbody R, Schroder M, et al. The human adaptor SARM negatively regulates adaptor protein TRIF-dependent Toll-like receptor signaling. Nat Immunol, 2006, 7: 1074–1081, 10.1038/ni1382, 16964262, 1:CAS:528:DC%2BD28Xps1yntbk%3D
Burns K, Clatworthy J, Martin L, et al. Tollip, a new component of the IL-1RI pathway, links IRAK to the IL-1 receptor. Nat Cell Biol, 2000, 2: 346–351, 10.1038/35014038, 10854325, 1:CAS:528:DC%2BD3cXlt1Sls7k%3D
Bulut Y, Faure E, Thomas L, et al. Cooperation of Toll-like receptor 2 and 6 for cellular activation by soluble tuberculosis factor and Borrelia burgdorferi outer surface protein A lipoprotein: Role of Toll-interacting protein and IL-1 receptor signaling molecules in Toll-like receptor 2 signaling. J Immunol, 2001, 167: 987–994, 11441107, 1:CAS:528:DC%2BD3MXotFWktLs%3D
Zhang G, Ghosh S. Negative regulation of toll-like receptor-mediated signaling by Tollip. J Biol Chem, 2002, 277: 7059–7065, 10.1074/jbc.M109537200, 11751856, 1:CAS:528:DC%2BD38XitV2murw%3D
Wang Y, Tang Y, Teng L, et al. Association of beta-arrestin and TRAF6 negatively regulates Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol, 2006, 7: 139–147, 10.1038/ni1294, 16378096, 1:CAS:528:DC%2BD28Xlt1ersw%3D%3D
Kawagoe T, Takeuchi O, Takabatake Y, et al. TANK is a negative regulator of Toll-like receptor signaling and is critical for the prevention of autoimmune nephritis. Nat Immunol, 2009, 10: 965–972, 10.1038/ni.1771, 19668221, 1:CAS:528:DC%2BD1MXps1ahtLk%3D
Zhao Q, Wang X, Nelin L D, et al. MAP kinase phosphatase 1 controls innate immune responses and suppresses endotoxic shock. J Exp Med, 2006, 203: 131–140, 10.1084/jem.20051794, 16380513, 1:CAS:528:DC%2BD28XhtVyru70%3D
Hammer M, Mages J, Dietrich H, et al. Dual specificity phosphatase 1 (DUSP1) regulates a subset of LPS-induced genes and protects mice from lethal endotoxin shock. J Exp Med, 2006, 203: 15–20, 10.1084/jem.20051753, 16380512, 1:CAS:528:DC%2BD28XhtVyrur4%3D
Chi H, Barry S P, Roth R J, et al. Dynamic regulation of pro- and anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) in innate immune responses. Proc Natl Acad Sci USA, 2006, 103: 2274–2279, 10.1073/pnas.0510965103, 16461893, 1:CAS:528:DC%2BD28XhslGjsbc%3D
Jeffrey K L, Brummer T, Rolph M S, et al. Positive regulation of immune cell function and inflammatory responses by phosphatase PAC-1. Nat Immunol, 2006, 7: 274–283, 10.1038/ni1310, 16474395, 1:CAS:528:DC%2BD28XhsVSrs70%3D
Xu H, An H, Hou J, et al. Phosphatase PTP1B negatively regulates MyD88- and TRIF-dependent proinflammatory cytokine and type I interferon production in TLR-triggered macrophages. Mol Immunol, 2008, 45: 3545–3552, 10.1016/j.molimm.2008.05.006, 18571728, 1:CAS:528:DC%2BD1cXosVajsLg%3D
An H, Zhao W, Hou J, et al. SHP-2 phosphatase negatively regulates the TRIF adaptor protein-dependent type I interferon and proinflammatory cytokine production. Immunity, 2006, 25: 919–928, 10.1016/j.immuni.2006.10.014, 17157040, 1:CAS:528:DC%2BD2sXisFeitw%3D%3D
Chuang T H, Ulevitch R J. Triad3A, an E3 ubiquitin-protein ligase regulating Toll-like receptors. Nat Immunol, 2004, 5: 495–502, 10.1038/ni1066, 15107846, 1:CAS:528:DC%2BD2cXjsV2gurk%3D
Wang Y, Chen T, Han C, et al. Lysosome-associated small Rab GTPase Rab7b negatively regulates TLR4 signaling in macrophages by promoting lysosomal degradation of TLR4. Blood, 2007, 110: 962–971, 10.1182/blood-2007-01-066027, 17395780, 1:CAS:528:DC%2BD2sXos1Cqsbg%3D
Yao M, Liu X, Li D, et al. Late endosome/lysosome-localized Rab7b suppresses TLR9-initiated proinflammatory cytokine and type I IFN production in macrophages. J Immunol, 2009, 183: 1751–1758, 10.4049/jimmunol.0900249, 19587007, 1:CAS:528:DC%2BD1MXoslCrsr8%3D
Kinjyo I, Hanada T, Inagaki-Ohara K, et al. SOCS1/JAB is a negative regulator of LPS-induced macrophage activation. Immunity, 2002, 17: 583–591, 10.1016/S1074-7613(02)00446-6, 12433365, 1:CAS:528:DC%2BD38XovF2lsro%3D
Nakagawa R, Naka T, Tsutsui H, et al. SOCS-1 participates in negative regulation of LPS responses. Immunity, 2002, 17: 677–687, 10.1016/S1074-7613(02)00449-1, 12433373, 1:CAS:528:DC%2BD38XovF2ls7w%3D
Mansell A, Smith R, Doyle S L, et al. Suppressor of cytokine signaling 1 negatively regulates Toll-like receptor signaling by mediating Mal degradation. Nat Immunol, 2006, 7: 148–155, 10.1038/ni1299, 16415872, 1:CAS:528:DC%2BD28Xlt1ertg%3D%3D
Gingras S, Parganas E, de Pauw A, et al. Re-examination of the role of suppressor of cytokine signaling 1 (SOCS1) in the regulation of toll-like receptor signaling. J Biol Chem, 2004, 279: 54702–54707, 10.1074/jbc.M411043200, 15491990, 1:CAS:528:DC%2BD2cXhtVylurvF
Baetz A, Frey M, Heeg K, et al. Suppressor of cytokine signaling (SOCS) proteins indirectly regulate toll-like receptor signaling in innate immune cells. J Biol Chem, 2004, 279: 54708–54715, 10.1074/jbc.M410992200, 15491991, 1:CAS:528:DC%2BD2cXhtVylurjN
Boone D L, Turer E E, Lee E G, et al. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat Immunol, 2004, 5: 1052–1060, 10.1038/ni1110, 15334086, 1:CAS:528:DC%2BD2cXnvFCgsro%3D
Saitoh T, Yamamoto M, Miyagishi M, et al. A20 is a negative regulator of IFN regulatory factor 3 signaling. J Immunol, 2005, 174: 1507–1512, 15661910, 1:CAS:528:DC%2BD2MXlsFymsA%3D%3D
Bignell G R, Warren W, Seal S, et al. Identification of the familial cylindromatosis tumour-suppressor gene. Nat Genet, 2000, 25: 160–165, 10.1038/76006, 10835629, 1:CAS:528:DC%2BD3cXjvFOgt7g%3D
Kovalenko A, Chable-Bessia C, Cantarella G, et al. The tumour suppressor CYLD negatively regulates NF-kappaB signalling by deubiquitination. Nature, 2003, 424: 801–805, 10.1038/nature01802, 12917691, 1:CAS:528:DC%2BD3sXmt1ansbY%3D
Brummelkamp T R, Nijman S M, Dirac A M, et al. Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-kappaB. Nature, 2003, 424: 797–801, 10.1038/nature01811, 12917690, 1:CAS:528:DC%2BD3sXmt1antr4%3D
Trompouki E, Hatzivassiliou E, Tsichritzis T, et al. CYLD is a deubiquitinating enzyme that negatively regulates NF-kappaB activation by TNFR family members. Nature, 2003, 424: 793–796, 10.1038/nature01803, 12917689, 1:CAS:528:DC%2BD3sXmt1ansbc%3D
Yoshida H, Jono H, Kai H, et al. The tumor suppressor cylindromatosis (CYLD) acts as a negative regulator for toll-like receptor 2 signaling via negative cross-talk with TRAF6 AND TRAF7. J Biol Chem, 2005, 280: 41111–41121, 10.1074/jbc.M509526200, 16230348, 1:CAS:528:DC%2BD2MXht1Oqt7rP
Wang C, Chen T, Zhang J, et al. The E3 ubiquitin ligase Nrdp1’ preferentially’ promotes TLR-mediated production of type I interferon. Nat Immunol 2009, 10: 744–752, 10.1038/ni.1742, 19483718, 1:CAS:528:DC%2BD1MXms1alt7s%3D
Shi M, Deng W, Bi E, et al. TRIM30 alpha negatively regulates TLR-mediated NF-kappa B activation by targeting TAB2 and TAB3 for degradation. Nat Immunol, 2008, 9: 369–377, 10.1038/ni1577, 18345001, 1:CAS:528:DC%2BD1cXjtlGmsLY%3D
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
An, H., Qian, C. & Cao, X. Regulation of Toll-like receptor signaling in the innate immunity. Sci. China Life Sci. 53, 34–43 (2010). https://doi.org/10.1007/s11427-010-0011-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11427-010-0011-x