Abstract
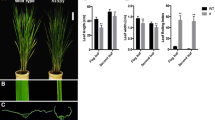
To understand the development of rice leaf blades, we identified a new rolled-leaf mutant, w32, from indica cultivar IR64 through EMS mutagenesis. The mutant showed a stable rolled-leaf phenotype throughout the life cycle. Two F2 populations were developed by crossing w32 to cultivar IR24 and PA64. Genetic analysis showed that the rolled-leaf phenotype was controlled by a single recessive gene. To determine the location of the gene, bulked segregant analysis was carried out using mutant and wild-type DNA pools and 1846 mutant-type F2 individuals derived from the cross w32/PA64 were genotyped to locate the gene on the short arm of chromosome 7. The rolled-leaf gene, tentatively named rl11(t), is likely a new gene as no other rolled-leaf genes have been identified near the region. By developing new SSR and InDel markers, the gene was delimited to a 52 kb region near the end of the short chromosome arm. Further fine mapping and cloning of the gene are currently underway.
Similar content being viewed by others
References
Zhu D F, Lin X Q, Cao W X. Comparison of leaf photosynthetic characteristics among rice hybrids with different leaf rolling index. (in Chinese) Acta Agro Sin, 2001, 273: 329–333
Li S G, Ma Y Q, He P, et al. Genetic analysis and mapping the flag leaf roll in rice (Oryza sativa L.). (in Chinese) J Sichuan Agri Univ, 1998, 16: 391–393
Shao Y J, Pan C H, Chen Z X, et al. Fine mapping of an incom-plete recessive gene for leaf rolling in rice (Oryza sativa L.). Chinese Sci Bull, 2005, 50: 2466–2472 10.1360/982005-999, 1:CAS:528:DC%2BD28Xks1eruw%3D%3D
Luo Z, Yang Z, Zhong B, et al. Genetic analysis and fine mapping of a dynamic rolled leaf gene, RL10(t), in rice (Oryza sativa L.). Genome, 2007, 50: 811–817 17893721, 10.1139/G07-064, 1:CAS:528:DC%2BD2sXhtlekt7%2FN
Yu D, Wu H B, Yang W T, et al. Genetic analysis and mapping of the unilateral rolled leaf trait of rice mutant B157. (in Chinese) Mol Plant Breed, 2008, 6: 220–226 1:CAS:528:DC%2BD1MXjvFGksLc%3D
Shao Y J, Chen Z X, Zhang Y F, et al. One major QTL mapping and physical map construction for rolled leaf in rice. (in Chinese) Acta Genet Sin, 2005, 32: 501–506 16018261, 1:CAS:528:DC%2BD28XjslSit7c%3D
Yi J C, Zhuang C X, Wang X J, et al. Genetic analysis and molecular mapping of a rolling leaf mutation gene in rice. J Integrative Plant Biol, 2007, 49: 1746–1753 10.1111/j.1744-7909.2007.00572.x, 1:CAS:528:DC%2BD1cXmsFynuw%3D%3D
Yan C J, Yan S, Zhang Z Q, et al. Genetic analysis and gene fine mapping for a rice novel mutant (rl9(t)) with rolling leaf character. Chinese Sci Bull, 2006, 51: 63–69 10.1007/s11434-005-1142-5
Yan S, Yan C J, Zeng X H, et al. Rolled leaf 9, encoding a GARP protein, regulates the leaf abaxial cell fate in rice. Plant Mol Biol, 2008, 68: 239–250 18594992, 10.1007/s11103-008-9365-x, 1:CAS:528:DC%2BD1cXhtVahu7%2FK
Wu J L, Lei C L, Wu C J, et al. Chemical- and irradiation-induced mutants of indica rice IR64. Plant Mol Biol, 2005, 59: 85–97 16217604, 10.1007/s11103-004-5112-0, 1:CAS:528:DC%2BD2MXhtV2ksbnK
Lu Y J, Zheng K L. A simple method for DNA extraction in rice. (in Chinese) Chin J Rice Sci, 1992, 6: 47–48
Yuan L P. Hybrid rice breeding for super high yield. (in Chinese) Hybrid rice, 1997, 12: 1–6
Xu L, Yang L, Huang H. Transcriptional post-transcriptional and post-translational regulations of gene expression during leaf polarity formation. Cell Res, 2007, 17: 512–519 17549070, 10.1038/cr.2007.45, 1:CAS:528:DC%2BD2sXms1Slsbg%3D
Yan S, Yan C J, Gu M H. Molecular mechanism of leaf development. (in Chinese) Hereditas, 2008, 30(9):1127–1135 18779169, 1:CAS:528:DC%2BD1MXos1Kls7c%3D
Waites R, Hudson A. Phantastica, a gene required for dorsoventrality of leaves in Antirrhinum majus. Development, 1995, 121: 2143–2154 1:CAS:528:DyaK2MXmsl2lu7k%3D
Emery J F, Floyd S K, Alvarez J, et al. Radial patterning of Arabidopsis shoots by class III HD-ZIP and KANADI genes. Curr Biol, 2003, 13: 1768–1774 14561401, 10.1016/j.cub.2003.09.035, 1:CAS:528:DC%2BD3sXot12itbg%3D
Siegfried K R, Eshed Y, Baum S F, et al. Members of the YABBY gene family specify abaxial cell fate in Arabidopsis. Development, 1999, 126: 4117–4128 10457020, 1:CAS:528:DyaK1MXmsleqsbY%3D
Kerstetter R A, Bollman K, Taylor A, et al. KANADI regulates organ polarity in Arabidopsis. Nature, 2001, 411: 706–709 11395775, 10.1038/35079629, 1:CAS:528:DC%2BD3MXksF2gur8%3D
Juarez M T, Kui J S, Thomas J, et al. microRNA-mediated repression of rolled leaf1 specifies maize leaf polarity. Nature, 2004, 428: 84–88 14999285, 10.1038/nature02363, 1:CAS:528:DC%2BD2cXhslCnsL8%3D
Kidner C A, Martienssen R A. Spatially restricted microRNA directs leaf polarity through ARGONAUTE1. Nature, 2004, 428: 81–84 14999284, 10.1038/nature02366, 1:CAS:528:DC%2BD2cXhslCnsLw%3D
Chitwood D H, Guo M J, Nogueira F TS, et al. Establishing leaf polarity: the role of small RNAs and positional signals in the shoot apex. Development, 2007, 134: 813–823 17251271, 10.1242/dev.000497, 1:CAS:528:DC%2BD2sXkt1Ogs7Y%3D
Zhao Y D, Christensen S K, Fankhauser C, et al. A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science, 2001, 291: 306–309 11209081, 10.1126/science.291.5502.306, 1:CAS:528:DC%2BD3MXktlKmsw%3D%3D
Tobena-Santamaria R, Bliek M, Ljung K, et al. FLOOZY of petunia is a flavin monooxygenase-like protein required for the specification of leaf and flower architecture. Genes Dev. 2002, 16: 753–763 11914280, 10.1101/gad.219502, 1:CAS:528:DC%2BD38XisVaisrw%3D
Fujino K, Matsuda Y, Ozawa K, et al. Narrow leaf 7 controls leaf shape mediated by auxin in rice. Mol Genet Genomics. 2008, 279: 499–507 18293011, 10.1007/s00438-008-0328-3, 1:CAS:528:DC%2BD1cXkvFWksrw%3D
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National High Technology Research and Development Program of China (Grant Nos. 2006AA10Z1E8 and 2006AA100101)
Rights and permissions
About this article
Cite this article
Shi, Y., Chen, J., Liu, W. et al. Genetic analysis and gene mapping of a new rolled-leaf mutant in rice (Oryza sativa L.). SCI CHINA SER C 52, 885–890 (2009). https://doi.org/10.1007/s11427-009-0109-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11427-009-0109-1