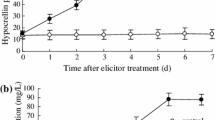
Abstract
Bax, a mammalian pro-apoptotic member of the Bcl-2 family, has been demonstrated to be a potential regulatory factor for plant secondary metabolite biosynthesis recently. To investigate the molecular mechanism of Bax-induced secondary metabolite biosynthesis, we determined the contents of nitric oxide (NO) of the transgenic Catharanthus roseus cells overexpressing a mouse Bax protein and checked the effects of NO specific scavenger 2,4-carboxyphenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPITO) on Bax-induced terpenoid indole alkaloid (TIA) production of the cells. The data showed that overexpression of the mouse Bax in C. roseus cells triggered NO generation of the cells. Treatment of cPITO not only inhibited the Bax-triggered NO burst but also suppressed the Bax-induced TIA production. The results indicated that the mouse Bax might activate the NO signaling in C. roseus cells and induce TIA production through the NO-dependent signal pathway in the cells. Furthermore, the activities of nitric oxide synthase (NOS) were significantly increased in the transgenic Bax cells as compared to those in the control cells, showing that the mouse Bax may induce NOS of C. roseus cells. Treatment of the transgenic Bax cells with NOS inhibitor PBITU blocked both Bax-induced NO generation and TIA production, which suggested that the mouse Bax might trigger NO generation and TIA production through NOS. However, the NOS-like activities and NO generation in the transgenic Bax cells did not match kinetically and the Bax-induced NOS-like activity was much later and lower than NO production. Moreover, the Bax-induced NO generation and TIA production were only partially inhibited by PBITU. Thus, our results suggested that the Bax-induced NO production and secondary metabolite biosynthesis in C. roseus cells was not entirely dependent on NOS or NOS-like enzymes.
Similar content being viewed by others
References
Sato F, Hashimoto T, Hachiya A, et al. Metabolic engineering of plant alkaloid biosynthesis. Proc Natl Acad Sci USA, 2001, 98: 367–372, 11134522, 10.1073/pnas.011526398, 1:CAS:528:DC%2BD3MXkslOnuw%3D%3D
Verpoorte R, van der Heijden R, Van Gulik W, et al. Plant biotechnology for the production of alkaloids: Present status and prospects. In: Brossi A, ed. The Alkaloids. Vol 40. New York: Academic Press, 1991. 2–187
Capell T, Christou P. Progress in plant metabolic engineering. Curr Opin Biotechnol, 2004, 15: 148–154, 15081054, 10.1016/j.copbio.2004.01.009, 1:CAS:528:DC%2BD2cXjtVCnuro%3D
Buchanan B B, Gurissem W, Jones R L, eds. Biochemistry and molecular biology of plants. 1st ed. USA: American Society of Plant Physiologists, 2000. 1250–1316
Rothe G, Hachiya A, Yamada Y, et al., Alkaloids in plants and root cultures of Atropa belladonna overexpressing putrescine N-methyltransferase. J Exp Bot, 2003, 54(390): 2065–2070, 12885861, 10.1093/jxb/erg227, 1:CAS:528:DC%2BD3sXns1Slu74%3D
Moyano E, Jouhikainen K, Tammela P, et al. Effect of pmt gene overexpression on tropane alkaloid production in transformed root cultures of Datura metel and Hyoscyamus muticus. J Exp Bot, 2003, 54(381): 203–211, 12493848, 10.1093/jxb/54.381.203, 1:CAS:528:DC%2BD3sXhtlOiuro%3D
Hahlbrock K, Bednarek P, Ciolkowski I, et al. Non-self recognition, transcriptional reprogramming, and secondary metabolite accumulation during plant/pathogen interactions. Proc Natl Acad Sci USA, 2003, 100s: 14569–14576, 10.1073/pnas.0831246100
Broun P, SomervIlle C. Progress in plant metabolic engineering. Proc Natl Acad Sci USA, 2001, 16: 8925–8927, 10.1073/pnas.171310598
Xu J, Dong J. Enhancing terpeniod indole alkaloid production by inducible expression of mammalian Bax in Catharanthus roseus cells. Sci China Ser C-Life Sci, 2007, 50(2): 234–241, 10.1007/s11427-007-0030-4, 1:CAS:528:DC%2BD2sXms1Ohs7c%3D
Neill S J, Desikan R, Hancock J T. Nitric oxide signaling in plants. New Phytologist, 2003, 159: 11–22, 10.1046/j.1469-8137.2003.00804.x, 1:CAS:528:DC%2BD3sXlslaltrs%3D
Delledonne M, Xia Y, Dixon R A, et al. Nitric oxide functions as a secondary signal in plant disease resistance. Nature, 1998, 394: 585–588, 9707120, 10.1038/29087, 1:CAS:528:DyaK1cXltlyktrc%3D
Modolo L V, Cunha F Q, Braga M R, et al. Nitric oxide synthase-mediated phytoalexin accumulation in soybean cotyledons in response to the Diaporthe phaseolorum f. sp. meridionalis elicitor. Plant Physiol, 2002, 130: 1288–1297, 12427995, 10.1104/pp.005850, 1:CAS:528:DC%2BD38XovVOms70%3D
Xu M, Dong J, Zhu M. Effect of nitric oxide on catharanthine production in Catharanthus roseus suspension cells. Biotechnol Bioeng, 2005, 89: 367–371, 15744842, 10.1002/bit.20334, 1:CAS:528:DC%2BD2MXhsFeluro%3D
Xu J, Dong J. Elicitor-induced nitric oxide burst is essential for triggering catharanthine synthesis in Catharanthus roseus suspension cells. Appl Microbiol Biotechnol, 2005, 67: 40–44, 15480633, 10.1007/s00253-004-1737-9, 1:CAS:528:DC%2BD2MXksFagsbY%3D
Xu J. Nitric oxide: a potential key-point of the signaling network leading to plant secondary metabolite biosynthesis. Prog Natl Sci (in press)
Xu J, Dong J, Zhang G. Enhancement of hypericin production and cell growth of Hypericum perforatum L. suspension cells by nitric oxide. Chin J Biotechnol, 2005, 21(1): 66–70
Baek D, Nam J, Koo Y D, et al. Bax-induced cell death of Arabidopsis is meditated through reactive oxygen-dependent and-independent processes. Plant Mol Biol, 2004, 56(1): 15–27, 15604726, 10.1007/s11103-004-3096-4, 1:CAS:528:DC%2BD2cXhtFWqsb3J
Murashige T, Skoog F. A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant, 1962, 15: 473–479, 10.1111/j.1399-3054.1962.tb08052.x, 1:CAS:528:DyaF3sXksFKm
Clark M S. Plant Molecular Biology—A Laboratory Manual. 1st ed. Berlin, Heidelberg: Springer-Verlag, 1979. 305–340
Xu M J, Dong J. Nitric oxide stimulates indole alkaloid production in Catharanthus roseus cell suspension cultures through a protein kinase-dependent signal pathway. Enzyme Microb Technol, 2005, 37: 49–53, 10.1016/j.enzmictec.2005.01.036, 1:CAS:528:DC%2BD2MXjvVKqs7o%3D
Romero-Puertas M C, Perazzolli M, Zago E D, et al. Nitric oxide signalling functions in plant-pathogen interactions. Cell Microbiol, 2004, 6(9): 795–803, 15272861, 10.1111/j.1462-5822.2004.00428.x, 1:CAS:528:DC%2BD2cXms1eitbc%3D
Durner J, Wendehenne D, Klessig D F. Defense gene induction in tobacco by nitric oxide, cyclic CMP and cyclic ADP-ribose. Proc Natl Acad Sci USA, 1998, 95: 10328–10333., 9707647, 10.1073/pnas.95.17.10328, 1:CAS:528:DyaK1cXlsFGgtrw%3D
Delledonne M, Zeier J, Marocco A, et al. Signal interaction between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proc Natl Acad Sci USA, 2001, 98: 13454–13459, 11606758, 10.1073/pnas.231178298, 1:CAS:528:DC%2BD3MXosFygurY%3D
Hu X Y, Neill S J, Cai W M. NO-mediated hypersensitive responses of rice suspension cultures induced by incompatible elicitor. Chin Sci Bull, 2003, 48: 358–363, 10.1360/03tb9076, 1:CAS:528:DC%2BD3sXis1eks78%3D
Rees D D, Cunha F Q, Assreuy J, et al. Sequential induction of nitric-oxide synthase by Corynebacterium parvum in different organs of the mouse. Br J Pharmacol, 1995, 114: 689–693, 7537593, 1:CAS:528:DyaK2MXjs1Witbs%3D
Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem, 1976, 72: 248–254, 942051, 10.1016/0003-2697(76)90527-3, 1:CAS:528:DyaE28XksVehtrY%3D
Simonen M, Keller H, Heim J. The BH3 domain of Bax is sufficient for interaction of Bax with itself and with other family members and it is required for induction of apoptosis. Eur J Biochem, 1997, 249: 85–91, 9363757, 10.1111/j.1432-1033.1997.t01-1-00085.x, 1:CAS:528:DyaK2sXmvVKntLk%3D
Zha H, Aime-Sempe C, Sato T, et al. Proapoptotic protein Bax heterodimerizes with Bcl-2 and homodimerizes with Bax via a novel domain (BH3) distinct from BH1 and BH2. J Biol Chem, 1997, 271: 7440–7444
Lacomme S, Cruz S S. Bax-induced cell death in tobacco is similar to the hypersensitive response. Proc Natl Acad Sci USA, 1999, 96: 7956–7961, 10393929, 10.1073/pnas.96.14.7956, 1:CAS:528:DyaK1MXltVOkurg%3D
Kiwai-Yamada M, Jin L, Yoshinage K, et al. Bax-induced plant cell death can be down-regulated by overexpression of Arabidopsis Bax Inhibitor-1 (AtBI-1). Proc Natl Acad Sci USA, 2001, 98: 12295–300, 10.1073/pnas.211423998
Xu M J, Dong J F, Zhu M Y. Nitric oxide mediates the fungal elicitor-induced hypericin production of Hypericum perforatum cell suspension cultures through a jasmonic acid-dependent signal pathway. Plant Physiol, 2005, 139: 991–998, 16169960, 10.1104/pp.105.066407, 1:CAS:528:DC%2BD2MXhtFCgsbzM
Xu M, Dong J, Zhu M. Involvement of NO in elicitor-induced PAL activation and Taxol synthesis stimulation of Taxus chinensis suspension cells. Chin Sci Bull, 2004, 49(10): 1038–1043, 10.1360/04wc0067, 1:CAS:528:DC%2BD2cXlvFCitrs%3D
Xu M, Dong J, Zhu M. Nitric oxide mediates the fungal elicitor-induced puerarin biosynthesis in Pueraria thomsonii Benth suspension cells through a salicylic acid (SA)-dependent and a jasmonic acid (JA)-dependent signal pathway. Sci China C-Life Sci, 2006, 49(4): 379–389, 16989284, 10.1007/s11427-006-2010-5, 1:CAS:528:DC%2BD28XhtVGitr3F
Kuo W N, Ku T W, Jones D L, et al. Nitric oxide synthase immunoreactivity in Baker’s yeasts, lobster and wheat germ. Biochem Arch, 1995, 11: 73–78, 1:CAS:528:DyaK2MXmslOrsbo%3D
Foissner I, Wendehenne D, Langebartels C, et al. In vivo imaging of an elicitor-induced nitric oxide burst in tobacco. Plant J, 2000, 23(6): 817–824, 10998192, 10.1046/j.1365-313X.2000.00835.x, 1:CAS:528:DC%2BD3cXnslWgt7k%3D
Xu M J, Dong J. O2− from elicitor-induced oxidative burst is necessary for triggering phenylalanine ammonia-lyase activation and catharanthine synthesis in Catharanthus roseus cell culture. Enzyme Microb Technol, 2005, 36: 280–284
Yuan Y, Li C, Hu Z et al. Signal transduction pathway for oxidative burst and taxol production in suspension cultures of Taxus chinensis vat mairei induced by oligosaccharide from Fusarium oxysprum. Enzyme Microb Technol, 2001, 29: 372–379, 10.1016/S0141-0229(01)00406-9, 1:CAS:528:DC%2BD3MXnt1Wgsr8%3D
Xu M J, Dong J. Nitric oxide mediates the fungal elicitor-induced Taxol biosynthesis of Taxus chinensis suspension cells through reactive oxygen species-dependent and-independent signal pathways. Chin Sci Bull, 2006, 51(16): 1967–1975, 10.1007/s11434-006-2081-5, 1:CAS:528:DC%2BD28XotlylurY%3D
Xu M J. Nitric oxide (NO)-induced plant secondary metabolite biosynthesis and its molecular basis. Dissertation for the Doctoral Degree. Hangzhou: Zhejiang University, 2005
Ribeiro E A, Cunha F Q, Tamashiro W M et al. Growth phase-dependent subcellular localization of nitric oxide synthase in maize cells. FEBS Letters, 1999, 445: 283–286, 10094473, 10.1016/S0014-5793(99)00138-6, 1:CAS:528:DyaK1MXhs1Oiur8%3D
Cueto M, Hernandez-Perera O, Martin R, et al. Presence of nitric oxide synthase activity in roots and nodules of Lupinus albus. FEBS Lett, 1996, 398(2–3): 159–164, 8977098, 10.1016/S0014-5793(96)01232-X, 1:CAS:528:DyaK28Xnt1Kqsb4%3D
Yamasaki H, Sakihama Y, Takahashi S. An alternative pathway for nitric oxide production in plants: New features of an old enzyme. Trends Plant Sci, 1999, 4: 128–129, 10322545, 10.1016/S1360-1385(99)01393-X
Yamasaki H, Sakihama Y. Simultaneous production of nitric oxide and peroxynitrite by plant nitrate reductase: in vitro evidence for the NR-dependent formation. FEBS Lett, 2000, 468: 89–92, 10683447, 10.1016/S0014-5793(00)01203-5, 1:CAS:528:DC%2BD3cXhtFamtb8%3D
Stöhr C, Strube F, Marx G, et al. A plasma membrane-bound enzyme of tobacco roots catalyses the formation of nitric oxide from nitrite. Planta, 2001, 212: 835–841, 11346959, 10.1007/s004250000447
Harrison R. Structure and function of xanthine oxidoreductase: Where are we now? Free Radical Biol Medicine, 2002, 33: 774–797, 10.1016/S0891-5849(02)00956-5, 1:CAS:528:DC%2BD38Xms1ymt7k%3D
Cooney R V, Harwood P J, Custer L J, et al. Light-mediated conversion of nitrogen dioxide to nitric oxide by carotenoids. Environ Health Perspectives, 1994, 102: 460–462, 10.2307/3432041, 1:CAS:528:DyaK2cXlvFOru7o%3D
Berthke P C, Badger M R, Jones R L. Apoplastic synthesis of nitric oxide by plant tissue. Plant Cell, 2004, 16: 332–3341, 10.1105/tpc.017822
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Natural Science Foundation of China (Grant No. 30572331), the Natural Science Foundation of Zhejiang Province, China (Grant No. 302785) and the Key Scientific Project of Zhejiang High Education Commission
Rights and permissions
About this article
Cite this article
Xu, M., Dong, J. Involvement of nitric oxide signaling in mammalian Bax-induced terpenoid indole alkaloid production of Catharanthus roseus cells. SCI CHINA SER C 50, 799–807 (2007). https://doi.org/10.1007/s11427-007-0096-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11427-007-0096-z