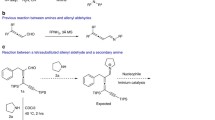
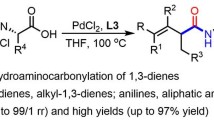
Abstract
1,3-Diene architectures are not only widely present in natural products, pharmaceuticals, and functional organic materials but also serve as versatile building blocks to furnish important functionalized molecules in synthetic chemistry due to conjugated repeating C=C units. Accordingly, various strategies to access substituted 1,3-dienes in a stereoselective manner have been developed. However, chemo-, regio- and stereoselective synthesis of highly substituted 1,3-dienes still remains elusive and challenging. Readily available propargylic esters have emerged as an appealing class of synthetic intermediates for accessing functionalized 1,3-dienes, especially challenging tri- or tetrasubstituted variants, via transition-metal catalysis, including electrophilic metal and redox neutral catalysis. This review, for the first time, systematically highlights recent advances in transition-metal catalyzed synthesis of substituted 1,3-dienes from propargylic esters, discusses the mechanisms and synthetic utilities, and gives the remaining challenges and potential opportunities in this field.
Similar content being viewed by others
References
Harned AM, Volp KA. Nat Prod Rep, 2011, 28: 1790–1810
Su Y, Li B, Xu H, Lu C, Wang S, Chen B, Wang Z, Wang W, Otake K, Kitagawa S, Huang L, Gu C. J Am Chem Soc, 2022, 144: 18218–18222
Mandal AK, Schneekloth, JS, Crews CM. Org Lett, 2005, 7: 3645–3648
Wang Y, Zhang X, Kong Y, Yang WL, Xu Z, Cheng J, Shao X, Xu X, Li Z. J Agric Food Chem, 2023, 71: 11332–11340
Harmon NM, Poe MM, Huang X, Singh R, Foust BJ, Hsiao CHC, Wiemer DF, Wiemer AJ. ACS Med Chem Lett, 2022, 13: 164–170
Tan X, Gao S, Yang C, Lang Q, Ding X, Chen GQ, Zhang X. Sci China Chem, 2023, 66: 2847–2851
Hamel C, Prusov E, Gertsch J, Schweizer W, Altmann K. Angew Chem Int Ed, 2008, 47: 10081–10085
Fürstner A, Nevado C, Waser M, Tremblay M, Chevrier C, Teplý F, Aïssa C, Moulin E, Müller O. J Am Chem Soc, 2007, 129: 9150–9161
Li R, Li J, Chen X, Liu J, Wang X, Tang S. Adv Synth Catal, 2022, 364: 4260–4265
Houk KN. Acc Chem Res, 1975, 8: 361–369
Corey EJ. Angew Chem Int Ed, 2002, 41: 1650–1667
Nicolaou KC, Snyder SA, Montagnon T, Vassilikogiannakis G. Angew Chem Int Ed, 2002, 41: 1668–1698
Eschenbrenner-Lux V, Kumar K, Waldmann H. Angew Chem Int Ed, 2014, 53: 11146–11157
Liu L, Kim H, Xie Y, Farès C, Kaib PSJ, Goddard R, List B. J Am Chem Soc, 2017, 139: 13656–13659
Zhao Q, Li Y, Zhang Q, Cheng J, Li X. Angew Chem Int Ed, 2021, 60: 17608–17614
Kennedy CR, Zhong H, Joannou MV, Chirik PJ. Adv Synth Catal, 2020, 362: 404–416
Armengol-Relats H, Mato M, Echavarren AM. Angew Chem Int Ed, 2021, 60: 1916–1922
Tan W, Zhang JY, Gao CH, Shi F. Sci China Chem, 2023, 66: 966–992
Zhou YY, Uyeda C. Science, 2019, 363: 857–862
Corti V, Barløse CL, Østergaard NL, Kristensen A, Jessen NI, Jørgensen KA. J Am Chem Soc, 2023, 145: 1448–1459
Werth J, Uyeda C. Angew Chem Int Ed, 2018, 57: 13902–13906
Sun Z, Dai M, Ding C, Chen S, Chen LA. J Am Chem Soc, 2023, 145: 18115–18125
Cornil J, Guérinot A, Cossy J. Org Biomol Chem, 2015, 13: 4129–4142
Sargent BT, Alexanian EJ. J Am Chem Soc, 2017, 139: 12438–12440
Szudkowska-Frątczak J, Marciniec B, Hreczycho G, Kubicki M, Pawluć P. Org Lett, 2015, 17: 2366–2369
Luo SXL, Cannon JS, Taylor BLH, Engle KM, Houk KN, Grubbs RH. J Am Chem Soc, 2016, 138: 14039–14046
Sit MK, Cao HH, Wu YD, Yip TC, Bendel LE, Zhang W, Dai WM. Org Lett, 2023, 25: 1633–1637
Funk TW, Efskind J, Grubbs RH. Org Lett, 2005, 7: 187–190
Li G, Huo X, Jiang X, Zhang W. Chem Soc Rev, 2020, 49: 2060–2118
Perry GJP, Jia T, Procter DJ. ACS Catal, 2020, 10: 1485–1499
Yang H, Yang ZQ, Zhang SZ, Zhang WW, Gu Q, You SL. Sci China Chem, 2023, 66: 2842–2846
Xiong Y, Zhang G. J Am Chem Soc, 2018, 140: 2735–2738
Sardini SR, Brown MK. J Am Chem Soc, 2017, 139: 9823–9826
Guo W, Wang Q, Zhu J. Angew Chem Int Ed, 2021, 60: 4085–4089
Hoffmann HMR. Angew Chem Int Ed, 1969, 8: 556–577
François B, Eberlin L, Berrée F, Whiting A, Carboni B. Eur J Org Chem, 2020, 2020: 3282–3293
Ghosh T, Gingrich HL, Kam CK, Mobraaten EC, Jones Jr. M. J Am Chem Soc, 1991, 113: 1313–1318
Johannsen M, Anker Jørgensen K. Tetrahedron, 1996, 52: 7321–7328
Kim HJ, Ruszczycky MW, Choi SH, Liu YN, Liu HW. Nature, 2011, 473: 109–112
Kelly WL. Org Biomol Chem, 2008, 6: 4483–4493
Stocking EM, Williams RM. Angew Chem Int Ed, 2003, 42: 3078–3115
Wang H, Zou Y, Li M, Tang Z, Wang J, Tian Z, Strassner N, Yang Q, Zheng Q, Guo Y, Liu W, Pan L, Houk KN. Nat Chem, 2023, 15: 177–184
Briou B, Améduri B, Boutevin B. Chem Soc Rev, 2021, 50: 11055–11097
Raynaud J, Wu JY, Ritter T. Angew Chem Int Ed, 2012, 51: 11805–11808
Han Y, Liu Z, Zhao Z, Liu B, Cui D. Organometallics, 2022, 41: 1412–1418
Leicht H, Göttker-Schnetmann I, Mecking S. J Am Chem Soc, 2017, 139: 6823–6826
Ruan XY, Zhang T, Li WA, Yin YZ, Han ZY, Gong LZ. Sci China Chem, 2022, 65: 863–869
Liao L, Guo R, Zhao X. Angew Chem Int Ed, 2017, 56: 3201–3205
Peng S, Yang J, Liu G, Huang Z. Sci China Chem, 2019, 62: 336–340
Yang Y, Li HX, Zhu TY, Zhang ZY, Yu ZX. J Am Chem Soc, 2023, 145: 17087–17095
Tortajada A, Ninokata R, Martin R. J Am Chem Soc, 2018, 140: 2050–2053
Liu S, Zhang D, Xiao M, Pu C, Zhang X, Yang X, Zhang T, Bai R. Org Chem Front, 2023, 10: 181–188
Korkis SE, Burns DJ, Lam HW. J Am Chem Soc, 2016, 138: 12252–12257
Yang HY, Lin LQ, Li NQ, Ren ZH, Guan ZH. Sci China Chem, 2023, 66: 1474–1481
Hubert P, Seibel E, Beemelmanns C, Campagne J, de Figueiredo RM. Adv Synth Catal, 2020, 362: 5532–5575
Negishi E, Huang Z, Wang G, Mohan S, Wang C, Hattori H. Acc Chem Res, 2008, 41: 1474–1485
Kenar JA, Havrilla CM, Porter NA, Guyton JR, Brown SA, Klemp KF, Selinger E. Chem Res Toxicol, 1996, 9: 737–744
Xu G, Xu J, Xu H, Cui X, Shu X. Chin J Org Chem, 2023, 43: 1899–1933
Soengas RG, Rodríguez-Solla H. Molecules, 2021, 26: 249
Paolis M, Chataigner I, Maddaluno J. Top Curr Chem, 2012, 327: 87–146
Pyziak J, Walkowiak J, Marciniec B. Chem Eur J, 2017, 23: 3502–3541
Diver ST, Giessert AJ. Chem Rev, 2004, 104: 1317–1382
de Haro T, Gómez-Bengoa E, Cribiú R, Huang X, Nevado C. Chem Eur J, 2012, 18: 6811–6824
Huang X, de Haro T, Nevado C. Chem Eur J, 2009, 15: 5904–5908
Green NJ, Willis AC, Sherburn MS. Angew Chem Int Ed, 2016, 55: 9244–9248
Rivera-Chao E, Fañanás-Mastral M. Angew Chem Int Ed, 2018, 57: 9945–9949
Rivera-Chao E, Fañanás-Mastral M. Angew Chem Int Ed, 2021, 60: 16922–16927
Cai H, Tu YQ, Lu K, Chen QL, Zhang FM, Zhang XM, Pan YJ, Yan ZB. Sci China Chem, 2023, 66: 2791–2796
Dutta S, Shandilya S, Yang S, Gogoi MP, Gandon V, Sahoo AK. Nat Commun, 2022, 13: 1360
Guo K, Kleij AW. Angew Chem Int Ed, 2021, 60: 4901–4906
Baek Y, Cheong K, Ko GH, Han GU, Han SH, Kim D, Lee K, Lee PH. J Am Chem Soc, 2020, 142: 9890–9895
Ping Y, Zhang S, Chang T, Wang J. J Org Chem, 2019, 84: 8275–8283
Yu H, Yu B, Zhang H, Huang H. Org Lett, 2021, 23: 3891–3896
Huang F, Huang Z, Liu G, Huang Z. Org Lett, 2022, 24: 5486–5490
Chen Y, Zhu K, Huang Q, Lu Y. Chem Sci, 2021, 12: 13564–13571
Sasaki Y, Horita Y, Zhong C, Sawamura M, Ito H. Angew Chem Int Ed, 2011, 50: 2778–2782
Zheng C, Wang D, Stahl SS. J Am Chem Soc, 2012, 134: 16496–16499
Stille JK, Groh BL. J Am Chem Soc, 1987, 109: 813–817
Karabelas K, Hallberg A. J Org Chem, 1988, 53: 4909–4914
Shen C, Zhu Y, Shen W, Jin S, Zhong G, Luo S, Xu L, Zhong L, Zhang J. Org Chem Front, 2022, 9: 2109–2115
Jin L, Zhang P, Li Y, Yu X, Shi BF. J Am Chem Soc, 2021, 143: 12335–12344
Hu T, Li M, Zhao Q, Feng C, Lin G. Angew Chem Int Ed, 2018, 57: 5871–5875
Olivares AM, Weix DJ. J Am Chem Soc, 2018, 140: 2446–2449
Liu Y, Wang L, Deng L. J Am Chem Soc, 2016, 138: 112–115
Liu J, Yang J, Baumann W, Jackstell R, Beller M. Angew Chem Int Ed, 2019, 58: 10683–10687
Li Y, Wu J, Li H, Sun Q, Xiong L, Yin G. Org Chem Front, 2021, 8: 628–634
Zhou P, Jiang H, Huang L, Li X. Chem Commun, 2011, 47: 1003–1005
Kakiuchi F, Uetsuhara T, Tanaka Y, Chatani N, Murai S. J Mol Catal A-Chem, 2002, 182–183: 511–514
Neisius N, Plietker B. Angew Chem Int Ed, 2009, 48: 5752–5755
Hou CJ, Schuppe AW, Knippel JL, Ni AZ, Buchwald SL. Org Lett, 2021, 23: 8816–8821
Zhu C, Yang B, Jiang T, Bäckvall J. Angew Chem Int Ed, 2015, 54: 9066–9069
Hampton CS, Harmata M. J Org Chem, 2016, 81: 4807–4822
Parisotto S, Palagi L, Prandi C, Deagostino A. Chem Eur J, 2018, 24: 5484–5488
Brown RW, Zamani F, Gardiner MG, Yu H, Pyne SG, Hyland CJT. Chem Sci, 2019, 10: 9051–9056
Yoshida M, Gotou T, Ihara M. Chem Commun, 2004, 1124
O’Broin CQ, Guiry PJ. J Org Chem, 2020, 85: 10321–10333
Niu B, Wei Y, Shi M. Org Chem Front, 2021, 8: 3475–3501
Dai M, Sun Z, Chen L. Angew Chem Int Ed, 2022, 61: e202203835
Ljungdahl N, Kann N. Angew Chem Int Ed, 2009, 48: 642–644
Zhang DY, Hu XP. Tetrahedron Lett, 2015, 56: 283–295
Zhu F, Li CX, Wu ZL, Cai T, Wen W, Guo QX. Nat Commun, 2022, 13: 7290
Hu Q, He Z, Peng L, Guo C. Nat Synth, 2022, 1: 322–331
Ye J, Ma S. Org Chem Front, 2014, 1: 1210–1224
Neff RK, Frantz DE. ACS Catal, 2014, 4: 519–528
Ma S. Eur J Org Chem, 2004, 2004: 1175–1183
Teng S, Chi YR, Zhou JS. Angew Chem Int Ed, 2021, 60: 4491–4495
Wang H, Qian H, Zhang J, Ma S. J Am Chem Soc, 2022, 144: 12619–12626
Xu X, Wang M, Peng L, Guo C. J Am Chem Soc, 2022, 144: 21022–21029
Guo LN, Duan XH, Liang YM. Acc Chem Res, 2011, 44: 111–122
Li G, Zhang G, Zhang L. J Am Chem Soc, 2008, 130: 3740–3741
Wang Z, Ying A, Fan Z, Hervieu C, Zhang L. ACS Catal, 2017, 7: 3676–3680
Hardin AR, Sarpong R. Org Lett, 2007, 9: 4547–4550
Shu XZ, Shu D, Schienebeck CM, Tang W. Chem Soc Rev, 2012, 41: 7698–7711
Soriano E, Marco-Contelles J. Chem Eur J, 2008, 14: 6771–6779
Rojas AFL, Kyne SH, Chan PWH. Acc Chem Res, 2023, 56: 1406–1420
Wang S. Tetrahedron Lett, 2018, 59: 1317–1327
Jiang J, Liu Y, Hou C, Li Y, Luan Z, Zhao C, Ke Z. Org Biomol Chem, 2016, 14: 3558–3563
Correa A, Marion N, Fensterbank L, Malacria M, Nolan S, Cavallo L. Angew Chem Int Ed, 2008, 47: 718–721
Johansson MJ, Gorin DJ, Staben ST, Toste FD. J Am Chem Soc, 2005, 127: 18002–18003
Shi X, Gorin DJ, Toste FD. J Am Chem Soc, 2005, 127: 5802–5803
Brambilla E, Pirovano V, Giannangeli M, Abbiati G, Caselli A, Rossi E. Org Chem Front, 2019, 6: 3078–3084
Prasad BAB, Yoshimoto FK, Sarpong R. J Am Chem Soc, 2005, 127: 12468–12469
Schwier T, Sromek AW, Yap DML, Chernyak D, Gevorgyan V. J Am Chem Soc, 2007, 129: 9868–9878
Marion N, Díez-González S, de Frémont P, Noble AR, Nolan SP. Angew Chem Int Ed, 2006, 45: 3647–3650
Zheng TL, Liu SZ, Huo CY, Li J, Wang BW, Jin DP, Cheng F, Chen XM, Zhang XM, Xu XT, Wang SH. CCS Chem, 2021, 3: 2795–2802
Wang S, Zhang L. Org Lett, 2006, 8: 4585–4587
Dudnik AS, Schwier T, Gevorgyan V. Tetrahedron, 2009, 65: 1859–1870
Dudnik AS, Schwier T, Gevorgyan V. J Organomet Chem, 2009, 694: 482–485
Garayalde D, Gómez-Bengoa E, Huang X, Goeke A, Nevado C. J Am Chem Soc, 2010, 132: 4720–4730
Shiroodi RK, Dudnik AS, Gevorgyan V. J Am Chem Soc, 2012, 134: 6928–6931
Cho EJ, Lee D. Adv Synth Catal, 2008, 350: 2719–2723
Cho EJ. Chem Eur J, 2012, 18: 4495–4498
Wang Y, Lu B, Zhang L. Chem Commun, 2010, 46: 9179–9181
Ambrogio I, Cacchi S, Fabrizi G, Prastaro A. Tetrahedron, 2009, 65: 8916–8929
Nemoto T, Zhao Z, Yokosaka T, Suzuki Y, Wu R, Hamada Y. Angew Chem Int Ed, 2013, 52: 2217–2220
Daniels DSB, Jones AS, Thompson AL, Paton RS, Anderson EA. Angew Chem Int Ed, 2014, 53: 1915–1920
Liu XN, Guo WL, Hou CJ, Hu XP. Synth Commun, 2013, 43: 2622–2626
Locascio TM, Tunge JA. Chem Eur J, 2016, 22: 18140–18146
Ishida N, Hori Y, Okumura S, Murakami M. J Am Chem Soc, 2019, 141: 84–88
O’Broin CQ, Guiry PJ. Org Lett, 2020, 22: 879–883
Ishida N, Kamino Y, Murakami M. Synlett, 2021, 32: 1621–1624
Zhang J, Chang X, Xu X, Wang H, Peng L, Guo C. Nat Commun, 2022, 13: 7049
Clark DA, Kulkarni AA, Kalbarczyk K, Schertzer B, Diver ST. J Am Chem Soc, 2006, 128: 15632–15636
Hiroi K, Kato F, Oguchi T, Saito S, Sone T. Tetrahedron Lett, 2008, 49: 3567–3569
Bray CVL, Dérien S, Dixneuf P. Angew Chem Int Ed, 2009, 48: 1439–1442
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22071111), the Jiangsu Specially Appointed Professor Plan, the Natural Science Foundation of Jiangsu Province (BK20201368, BK20220409), and the Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX23_1683).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Dai, M., Song, L. & Chen, LA. Stereoselective synthesis of substituted 1,3-dienes from propargylic esters: electrophilic-metal or redox catalysis?. Sci. China Chem. (2024). https://doi.org/10.1007/s11426-023-1925-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11426-023-1925-4