Abstract
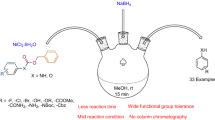
A simple electrochemically mediated method for the conversion of nitroarenes to aryl boronic esters is presented. Electrochemical borylation of a diverse range of nitroarenes, including the late-stage borylation of bioactive molecules, is furnished at room temperature under simple conditions, thereby demonstrating the broad utility and functional-group tolerance of this protocol. This transformation provides a convenient and practical access to aryl boronic esters from widely available nitroarenes, which would significantly streamline the synthetic process of diverse functionalized arenes.

Similar content being viewed by others
References
Ono N. The Nitro Group in Organic Synthesis. New York: Wiley-VCH, 2001
Booth G. Nitro Compounds, Aromatic. New York: Wiley-VCH, 2000
Bunnett JF, Zahler RE. Chem Rev, 1951, 49: 273–412
Makosza M, Winiarski J. Acc Chem Res, 1987, 20: 282–289
Caron L, Campeau LC, Fagnou K. Org Lett, 2008, 10: 4533–4536
Sengupta S, Das P. Org Biomol Chem, 2021, 19: 8409–8424
Zheng X, Ding J, Chen J, Gao W, Liu M, Wu H. Org Lett, 2011, 13: 1726–1729
Zhang J, Chen J, Liu M, Zheng X, Ding J, Wu H. Green Chem, 2012, 14: 912–916
Yu XC, Li B, Yu BH, Xu Q. Chin Chem Lett, 2013, 24: 605–608
Ryan SJ, Schimler SD, Bland DC, Sanford MS. Org Lett, 2015, 17: 1866–1869
Xuan M, Lu C, Lin BL. Chin Chem Lett, 2020, 31: 84–90
Yadav MR, Nagaoka M, Kashihara M, Zhong RL, Miyazaki T, Sakaki S, Nakao Y. J Am Chem Soc, 2017, 139: 9423–9426
Asahara KK, Okita T, Saito AN, Muto K, Nakao Y, Yamaguchi J. Org Lett, 2019, 21: 4721–4724
Chen K, Chen W, Yi X, Chen W, Liu M, Wu H. Chem Commun, 2019, 55: 9287–9290
Kashihara M, Zhong RL, Semba K, Sakaki S, Nakao Y. Chem Commun, 2019, 55: 9291–9294
Okita T, Asahara KK, Muto K, Yamaguchi J. Org Lett, 2020, 22: 3205–3208
Feng B, Yang Y, You J. Chem Commun, 2020, 56: 790–793
Feng B, Yang Y, You J. Chem Sci, 2020, 11: 6031–6035
Zhou F, Zhou F, Su R, Yang Y, You J. Chem Sci, 2020, 11: 7424–7428
Li Z, Peng Y, Wu T. Org Lett, 2021, 23: 881–885
Inoue F, Kashihara M, Yadav MR, Nakao Y. Angew Chem Int Ed, 2017, 56: 13307–13309
Chen W, Chen K, Chen W, Liu M, Wu H. ACS Catal, 2019, 9: 8110–8115
Kashihara M, Yadav MR, Nakao Y. Org Lett, 2018, 20: 1655–1658
Peng L, Hu Z, Tang Z, Jiao Y, Xu X. Chin Chem Lett, 2019, 30: 1481–1487
Muto K, Okita T, Yamaguchi J. ACS Catal, 2020, 10: 9856–9871
Kashihara M, Nakao Y. Acc Chem Res, 2021, 54: 2928–2935
Hall DG. Boronic Acids: Preparation and Applications in Organic Synthesis Medicine and Materials. 2nd ed. Weinheim: Wiley-VCH, 2011
Mo F, Jiang Y, Qiu D, Zhang Y, Wang J. Angew Chem Int Ed, 2010, 49: 1846–1849
Mo F, Qiu D, Zhang L, Wang J. Chem Rev, 2021, 121: 5741–5829
Cao ZC, Li XL, Luo QY, Fang H, Shi ZJ. Org Lett, 2018, 20: 1995–1998
Shiozuka A, Sekine K, Kuninobu Y. Org Lett, 2021, 23: 4774–4778
Ji S, Qin S, Yin C, Luo L, Zhang H. Org Lett, 2022, 24: 64–68
Shiozuka A, Sekine K, Toki T, Kawashima K, Mori T, Kuninobu Y. Org Lett, 2022, 24: 4281–4285
Tobisu M, Nakamura K, Chatani N. J Am Chem Soc, 2014, 136: 5587–5590
Zhang H, Hagihara S, Itami K. Chem Eur J, 2015, 21: 16796–16800
Hu J, Sun H, Cai W, Pu X, Zhang Y, Shi Z. J Org Chem, 2016, 81: 14–24
Mfuh AM, Doyle JD, Chhetri B, Arman HD, Larionov OV. J Am Chem Soc, 2016, 138: 2985–2988
Jin S, Dang HT, Haug GC, He R, Nguyen VD, Nguyen VT, Arman HD, Schanze KS, Larionov OV. J Am Chem Soc, 2020, 142: 1603–1613
Kong X, Lin L, Chen Q, Xu B. Org Chem Front, 2021, 8: 702–707
Ma Y, Pang Y, Chabbra S, Reijerse EJ, Schnegg A, Niski J, Leutzsch M, Cornella J. Chem Eur J, 2020, 26: 3738–3743
Du L, Sun L, Zhang H. Chem Commun, 2022, 58: 1716–1719
Wang M, Shi Z. Chem Rev, 2020, 120: 7348–7398
Tian YM, Guo XN, Braunschweig H, Radius U, Marder TB. Chem Rev, 2021, 121: 3561–3597
Francke R, Little RD. Chem Soc Rev, 2014, 43: 2492–2521
Yan M, Kawamata Y, Baran PS. Chem Rev, 2017, 117: 13230–13319
Moeller KD. Chem Rev, 2018, 118: 4817–4833
Yuan Y, Lei A. Acc Chem Res, 2019, 52: 3309–3324
Xiong P, Xu HC. Acc Chem Res, 2019, 52: 3339–3350
Röckl JL, Pollok D, Franke R, Waldvogel SR. Acc Chem Res, 2020, 53: 45–61
Ackermann L. Acc Chem Res, 2020, 53: 84–104
Jiao KJ, Xing YK, Yang QL, Qiu H, Mei TS. Acc Chem Res, 2020, 53: 300–310
Siu JC, Fu N, Lin S. Acc Chem Res, 2020, 53: 547–560
Wang F, Stahl SS. Acc Chem Res, 2020, 53: 561–574
Hong J, Liu Q, Li F, Bai G, Liu G, Li M, Nayal OS, Fu X, Mo F. Chin J Chem, 2019, 37: 347–351
Barton LM, Chen L, Blackmond DG, Baran PS. Proc Natl Acad Sci USA, 2021, 118: e2109408118
Wang B, Peng P, Ma W, Liu Z, Huang C, Cao Y, Hu P, Qi X, Lu Q. J Am Chem Soc, 2021, 143: 12985–12991
Dai JJ, Teng XX, Fang W, Xu J, Xu HJ. Chin Chem Lett, 2022, 33: 1555–1558
Lu H, Geng Z, Li J, Zou D, Wu Y, Wu Y. Org Lett, 2016, 18: 2774–2776
Yang K, Zhou F, Kuang Z, Gao G, Driver TG, Song Q. Org Lett, 2016, 18: 4088–4091
Zhou Y, Zhou H, Liu S, Pi D, Shen G. Tetrahedron, 2017, 73: 3898–3904
Chen D, Zhou Y, Zhou H, Liu S, Liu Q, Zhang K, Uozumi Y. Synlett, 2018, 29: 1765–1768
Du HC, Simmons N, Faver JC, Yu Z, Palaniappan M, Riehle K, Matzuk MM. Org Lett, 2019, 21: 2194–2199
Hosoya H, Misal Castro LC, Sultan I, Nakajima Y, Ohmura T, Sato K, Tsurugi H, Suginome M, Mashima K. Org Lett, 2019, 21: 9812–9817
Kallitsakis MG, Ioannou DI, Terzidis MA, Kostakis GE, Lykakis IN. Org Lett, 2020, 22: 4339–4343
Wang B, Ma J, Ren H, Lu S, Xu J, Liang Y, Lu C, Yan H. Chin Chem Lett, 2022, 33: 2420–2424
Yan G, Huang D, Wu X. Adv Synth Catal, 2018, 360: 1040–1053
Friese FW, Studer A. Chem Sci, 2019, 10: 8503–8518
Liu Q, Zhang L, Mo F. Acta Chim Sin, 2020, 78: 1297–1308
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21602096).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest The authors declare no conflict of interest.
Additional information
Supporting information The supporting information is available online at http://chem.scichina.com and http://link.springer.com/journal/11426. The supporting materials are published as submitted, without typesetting or editing. The responsibility for scientific accuracy and content remains entirely with the authors.
Supplementary Information
Rights and permissions
About this article
Cite this article
Du, L., Zhang, B., Ji, S. et al. Electrochemical borylation of nitroarenes. Sci. China Chem. 66, 534–539 (2023). https://doi.org/10.1007/s11426-022-1470-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-022-1470-8