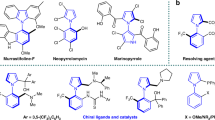
Abstract
Axially chiral molecules have been widely used in inorganic, material and medicinal chemistry. Compared with well-established N-heterocyclic carbene (NHC)-catalyzed asymmetric construction of centrally chiral molecules, NHC-catalyzed atroposelective synthesis of axially chiral molecules remains largely underdeveloped. Notably, alkynyl acyl azolium intermediates were commonly used in constructing a heteroaryl ring to furnish axially enantioenriched heteroaryl-aryls. The inherent character of the intermediates often led to react with sterically hindered substrates difficultly. Herein, we have successfully disclosed the atroposelective synthesis of axially chiral heteroaryl-aryls from sterically hindered enols through the use of chiral NHCs as catalysts for highly enantioselective Coates-Claisen rearrangements via catalytically generated α,β-unsaturated acyl azoliums. This approach will enable the concise synthesis of valuable tetra-ortho-substituted 2-pyrones in one step with good yield and chirality control.

Similar content being viewed by others
References
Jacobsen EN, Pfaltz A, Yamamoto H. Comprehensive Asymmetric Catalysis. Vols. I–III, Supplements I and II. New York: Springer, 1999
Carreira EM, Yamamoto H. Comprehensive Chirality. Vol. 1–9. London: Elsevier Science, 2012
The Nobel Prize in Chemistry 2021. NobelPrize.org. Nobel Prize Outreach AB 2021
Bugaut X, Glorius F. Chem Soc Rev, 2012, 41: 3511–3522
De Sarkar S, Biswas A, Samanta RC, Studer A. Chem Eur J, 2013, 19: 4664–4678
Ryan SJ, Candish L, Lupton DW. Chem Soc Rev, 2013, 42: 4906–4917
Hopkinson MN, Richter C, Schedler M, Glorius F. Nature, 2014, 510: 485–496
Flanigan DM, Romanov-Michailidis F, White NA, Rovis T. Chem Rev, 2015, 115: 9307–9387
Menon RS, Biju AT, Nair V. Chem Soc Rev, 2015, 44: 5040–5052
Chen XY, Liu Q, Chauhan P, Enders D. Angew Chem Int Ed, 2018, 57: 3862–3873
Mondal S, Yetra SR, Mukherjee S, Biju AT. Acc Chem Res, 2019, 52: 425–436
Chen XY, Gao ZH, Ye S. Acc Chem Res, 2020, 53: 690–702
Ohmiya H. ACS Catal, 2020, 10: 6862–6869
Chen X, Wang H, Jin Z, Chi YR. Chin J Chem, 2020, 38: 1167–1202
Barik S, Biju AT. Chem Commun, 2020, 56: 15484–15495
Das TK, Biju AT. Chem Commun, 2020, 56: 8537–8552
Biju AT. N-heterocyclic Carbenes in Organocatalysis. Weinheim: Wiley-VCH, 2019
Li T, Jin Z, Chi YR. Sci China Chem, 2022, 65: 210–223
Chen KQ, Sheng H, Liu Q, Shao PL, Chen XY. Sci China Chem, 2021, 64: 7–16
Lv J, Xu J, Pan X, Jin Z, Chi YR. Sci China Chem, 2021, 64: 985–990
Kumarasamy E, Raghunathan R, Sibi MP, Sivaguru J. Chem Rev, 2015, 115: 11239–11300
Bringmann G, Price Mortimer AJ, Keller PA, Gresser MJ, Garner J, Breuning M. Angew Chem Int Ed, 2005, 44: 5384–5427
Smyth JE, Butler NM, Keller PA. Nat Prod Rep, 2015, 32: 1562–1583
Shen D, Xu Y, Shi SL. J Am Chem Soc, 2019, 141: 14938–14945
Tang W, Zhang X. Chem Rev, 2003, 103: 3029–3070
Li Y, Kwong F, Yu W, Chan A. Coord Chem Rev, 2007, 251: 2119–2144
Parmar D, Sugiono E, Raja S, Rueping M. Chem Rev, 2014, 114: 9047–9153
Giacalone F, Gruttadauria M, Agrigento P, Noto R. Chem Soc Rev, 2012, 41: 2406–2447
Xiao Y, Sun Z, Guo H, Kwon O. Beilstein J Org Chem, 2014, 10: 2089–2121
Akiyama T, Mori K. Chem Rev, 2015, 115: 9277–9306
Clayden J, Moran WJ, Edwards PJ, LaPlante SR. Angew Chem Int Ed, 2009, 48: 6398–6401
Laplante SRD, Fader L, Fandrick KR, Fandrick DR, Hucke O, Kemper R, Miller SPF, Edwards PJ. J Med Chem, 2011, 54: 7005–7022
LaPlante SR, Edwards PJ, Fader LD, Jakalian A, Hucke O. ChemMedChem, 2011, 6: 505–513
McCormick MH, Stark WM, Pittenger GE, Pittenger RC, McGuire JM. Antibiot Annu, 1955, 3: 606–611
Kupchan SM, Britton RW, Ziegler MF, Gilmore CJ, Restivo RJ, Bryan RF. J Am Chem Soc, 1973, 95: 1335–1336
Hallock YF, Manfredi KP, Blunt JW, Cardellina Ii JH, Schaeffer M, Gulden KP, Bringmann G, Lee AY, Clardy J. J Org Chem, 1994, 59: 6349–6355
Bringmann G, Menche D. Acc Chem Res, 2001, 34: 615–624
Kozlowski MC, Morgan BJ, Linton EC. Chem Soc Rev, 2009, 38: 3193–3207
Hughes CC, Kauffman CA, Jensen PR, Fenical W. J Org Chem, 2010, 75: 3240–3250
Erbas-Cakmak S, Leigh DA, McTernan CT, Nussbaumer AL. Chem Rev, 2015, 115: 10081–10206
Collins BSL, Kistemaker JCM, Otten E, Feringa BL. Nat Chem, 2016, 8: 860–866
Takaishi K, Yasui M, Ema T. J Am Chem Soc, 2018, 140: 5334–5338
Sapotta M, Spenst P, Saha-Möller CR, Würthner F. Org Chem Front, 2019, 6: 892–899
For reviews, see: Song R, Xie Y, Jin Z, Chi YR. Angew Chem Int Ed, 2021, 60: 26026–26037
Wang J, Zhao C, Wang J. ACS Catal, 2021, 11: 12520–12531
Feng J, Du D. Tetrahedron, 2021, 100: 132456
Lu S, Poh SB, Zhao Y. Angew Chem Int Ed, 2014, 53: 11041–11045
Candish L, Levens A, Lupton DW. Chem Sci, 2015, 6: 2366–2370
Zhao C, Guo D, Munkerup K, Huang KW, Li F, Wang J. Nat Commun, 2018, 9: 611
Yang G, Guo D, Meng D, Wang J. Nat Commun, 2019, 10: 3062
Lu S, Ong JY, Yang H, Poh SB, Liew X, Seow CSD, Wong MW, Zhao Y. J Am Chem Soc, 2019, 141: 17062–17067
Lu S, Poh SB, Rong ZQ, Zhao Y. Org Lett, 2019, 21: 6169–6172
Xu K, Li W, Zhu S, Zhu T. Angew Chem Int Ed, 2019, 58: 17625–17630
Wu YT, Zhang R, Duan XY, Yu HF, Sun BY, Qi J. Chem Commun, 2020, 56: 9854–9857
Barik S, Shee S, Das S, Gonnade RG, Jindal G, Mukherjee S, Biju AT. Angew Chem Int Ed, 2021, 60: 12264–12268
Zhang CL, Gao YY, Wang HY, Zhou BA, Ye S. Angew Chem Int Ed, 2021, 60: 13918–13922
Li T, Mou C, Qi P, Peng X, Jiang S, Hao G, Xue W, Yang S, Hao L, Chi YR, Jin Z. Angew Chem Int Ed, 2021, 60: 9362–9367
Ma R, Wang X, Zhang Q, Chen L, Gao J, Feng J, Wei D, Du D. Org Lett, 2021, 23: 4267–4272
Jin J, Huang X, Xu J, Li T, Peng X, Zhu X, Zhang J, Jin Z, Chi YR. Org Lett, 2021, 23: 3991–3996
McGlacken GP, Fairlamb IJS. Nat Prod Rep, 2005, 22: 369–385
Tempone AG, Ferreira DD, Lima ML, Costa Silva TA, Borborema SET, Reimão JQ, Galuppo MK, Guerra JM, Russell AJ, Wynne GM, Lai RYL, Cadelis MM, Copp BR. Eur J Medicinal Chem, 2017, 139: 947–960
Al-Khdhairawi AAQ, Cordell GA, Thomas NF, Shivanagere Nagojappa NB, Weber JFF. Org Biomol Chem, 2019, 17: 8943–8957
Markó IE, Warriner SL, Augustyns B. Org Lett, 2000, 2: 3123–3125
Wang Y, Li H, Wang YQ, Liu Y, Foxman BM, Deng L. J Am Chem Soc, 2007, 129: 6364–6365
Meguro T, Chen S, Kanemoto K, Yoshida S, Hosoya T. Chem Lett, 2019, 48: 582–585
Darzi ER, Barber JS, Garg NK. Angew Chem Int Ed, 2019, 58: 9419–9424
Cole CJF, Fuentes L, Snyder SA. Chem Sci, 2020, 11: 2175–2180
Si XG, Zhang ZM, Zheng CG, Li ZT, Cai Q. Angew Chem Int Ed, 2020, 59: 18412–18417
Xu MM, You XY, Zhang YZ, Lu Y, Tan K, Yang L, Cai Q. J Am Chem Soc, 2021, 143: 8993–9001
Zhang Y, Huang J, Guo Y, Li L, Fu Z, Huang W. Angew Chem Int Ed, 2018, 57: 4594–4598
Wang G, Shi Q, Hu W, Chen T, Guo Y, Hu Z, Gong M, Guo J, Wei D, Fu Z, Huang W. Nat Commun, 2020, 11: 946
Wang G, Zhang QC, Wei C, Zhang Y, Zhang L, Huang J, Wei D, Fu Z, Huang W. Angew Chem Int Ed, 2021, 60: 7913–7919
Wang G, Zhang M, Guan Y, Zhang Y, Zheng P, Wei D, Fu Z, Chi YR, Huang W. Research, 2021, 2020: 8685436
Kaeobamrung J, Mahatthananchai J, Zheng P, Bode JW. J Am Chem Soc, 2010, 132: 8810–8812
Mahatthananchai J, Kaeobamrung J, Bode JW. ACS Catal, 2012, 2: 494–503
Lyngvi E, Bode JW, Schoenebeck F. Chem Sci, 2012, 3: 2346–2350
Kravina AG, Mahatthananchai J, Bode JW. Angew Chem Int Ed, 2012, 51: 9433–9436
Cheng J, Huang Z, Chi YR. Angew Chem Int Ed, 2013, 52: 8592–8596
Candish L, Lupton DW. J Am Chem Soc, 2013, 135: 58–61
Mahatthananchai J, Bode JW. Acc Chem Res, 2014, 47: 696–707
Yetra SR, Kaicharla T, Kunte SS, Gonnade RG, Biju AT. Org Lett, 2013, 15: 5202–5205
García-López JA, Greaney MF. Chem Soc Rev, 2016, 45: 6766–6798
Takikawa H, Nishii A, Sakai T, Suzuki K. Chem Soc Rev, 2018, 47: 8030–8056
Werz DB, Biju AT. Angew Chem Int Ed, 2020, 59: 3385–3398
Shi J, Li L, Li Y. Chem Rev, 2021, 121: 3892–4044
Fluegel LL, Hoye TR. Chem Rev, 2021, 121: 2413–2444
Roy T, Biju AT. Chem Commun, 2018, 54: 2580–2594
Bhojgude SS, Bhunia A, Biju AT. Acc Chem Res, 2016, 49: 1658–1670
Acknowledgements
This work was supported by the National Key R&D Program of China (2017YFA0204704), the National Natural Science Foundation of China (21602105), the General Program of Chongqing Natural Science Foundation Project (cstc2020jcyj-msxmX0712), Ningbo Natural Science Foundation (202003N4063), and the Natural Science Foundation of Jiangsu Province (BK20221309).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest The authors declare no conflict of interest.
Additional information
Supporting information The supporting information is available online at http://chem.scichina.com and http://link.springer.com/journal/11426. The supporting materials are published as submitted, without typesetting or editing. The responsibility for scientific accuracy and content remains entirely with the authors.
Rights and permissions
About this article
Cite this article
Wang, G., Huang, J., Zhang, L. et al. N-heterocyclic carbene-catalyzed atroposelective synthesis of axially chiral 5-aryl 2-pyrones from enals. Sci. China Chem. 65, 1953–1961 (2022). https://doi.org/10.1007/s11426-022-1327-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-022-1327-4