Abstract
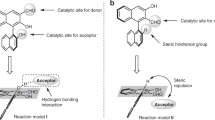
An N-heterocyclic carbene (NHC)-catalyzed enantioselective Mannich reaction of the remote γ-carbon of cyclopropylcarbaldehydes is disclosed for the first time. Diastereo- and enantiomerically enriched multicyclic δ-lactam compound is afforded as the main product from 8 possible stereo-specific isomers through dynamic kinetic asymmetric transformation (DYKAT) processes. Multiple chiral functional molecules can be afforded from the lactam products through simple protocols with retentions of the optical purities.

Similar content being viewed by others
References
For selected reviews, see (a) Danishefsky S. Acc Chem Res, 1979, 12: 66–72
Reissig HU, Zimmer R. Chem Rev, 2003, 103: 1151–1196
Carson CA, Kerr MA. Chem Soc Rev, 2009, 38: 3051–3060
Cavitt MA, Phun LH, France S. Chem Soc Rev, 2014, 43: 804–818
Schneider TF, Kaschel J, Werz DB. Angew Chem Int Ed, 2014, 53: 5504–5523
Werz DB, Biju AT. Angew Chem Int Ed, 2020, 59: 3385–3398
For selected examples, see: (g) Reissig HU, Hirsch E. Angew Chem Int Ed Engl, 1980, 19: 813–814
Brückner C, Reissig H-U. Angew Chem Int Ed, 1985, 24: 588–589
Kreft A, Lücht A, Grunenberg J, Jones PG, Werz DB. Angew Chem Int Ed, 2019, 58: 1955–1959
Petzold M, Jones PG, Werz DB. Angew Chem Int Ed, 2019, 58: 6225–6229
Xie MS, Zhao GF, Qin T, Suo YB, Qu GR, Guo HM. Chem Commun, 2019, 55: 1580–1583
For selected reviews on NHC organocatalysis, see: (a) Enders D, Niemeier O, Henseler A. Chem Rev, 2007, 107: 5606–5655
Biju AT, Kuhl N, Glorius F. Acc Chem Res, 2011, 44: 1182–1195
Bugaut X, Glorius F. Chem Soc Rev, 2012, 41: 3511–3522
Cohen DT, Scheidt KA. Chem Sci, 2012, 3: 53–57
Grossmann A, Enders D. Angew Chem Int Ed, 2012, 51: 314–325
Ryan SJ, Candish L, Lupton DW. Chem Soc Rev, 2013, 42: 4906–4917
Connon SJ. Angew Chem Int Ed, 2014, 53: 1203–1205
Hopkinson MN, Richter C, Schedler M, Glorius F. Nature, 2014, 510: 485–496
Mahatthananchai J, Bode JW. Acc Chem Res, 2014, 47: 696–707
Flanigan DM, Romanov-Michailidis F, White NA, Rovis T. Chem Rev, 2015, 115: 9307–9387
Menon RS, Biju AT, Nair V. Chem Soc Rev, 2015, 44: 5040–5052
Wang MH, Scheidt KA. Angew Chem Int Ed, 2016, 55: 14912–14922
Zhang C, Hooper JF, Lupton DW. ACS Catal, 2017, 7: 2583–2596
Murauski KJR, Jaworski AA, Scheidt KA. Chem Soc Rev, 2018, 47: 1773–1782
Chen X, Wang H, Jin Z, Chi YR. Chin J Chem, 2020, 38: 1167–1202
Chow KYK, Bode JW. J Am Chem Soc, 2004, 126: 8126–8127
(a)_Sohn SS, Bode JW. Angew Chem Int Ed, 2006, 45: 6021–6024
Bode JW, Sohn SS. J Am Chem Soc, 2007, 129: 13798–13799
Du D, Wang Z. Eur J Org Chem, 2008, 29: 4949–4954
Du D, Li L, Wang Z. J Org Chem, 2009, 74: 4379–4382
Li L, Du D, Ren J, Wang Z. Eur J Org Chem, 2011, 3: 614–618
Li GQ, Dai LX, You SL. Org Lett, 2009, 11: 1623–1625
Prieto L, Sánchez-Díez E, Uria U, Reyes E, Carrillo L, Vicario JL. Adv Synth Catal, 2017, 359: 1678–1683
Gao YY, Zhang CL, Dai L, Han YF, Ye S. Org Lett, 2021, 23: 1361–1366
Lv H, Mo J, Fang X, Chi YR. Org Lett, 2011, 13: 5366–5369
Li BS, Wang Y, Jin Z, Chi YR. Chem Sci, 2015, 6: 6008–6012
Li BS, Wang Y, Jin Z, Zheng P, Ganguly R, Chi YR. Nat Commun, 2015, 6: 6207–6211
Wang H, Jiang T, Xu MH. J Am Chem Soc, 2013, 135: 971–974
Wang H, Gu S, Yan Q, Ding L, Chen FE. Green Synth Catal, 2020, 1: 12–25
Dai L, Ye S. Org Lett, 2020, 22: 986–990
Breslow R. J Am Chem Soc, 1958, 80: 3719–3726
Kerr MS, Read de Alaniz J, Rovis T. J Am Chem Soc, 2002, 124: 10298–10299
He M, Struble JR, Bode JW. J Am Chem Soc, 2006, 128: 8418–8420
Enders D, Balensiefer T. Acc Chem Res, 2004, 37: 534–541
DiRocco DA, Rovis T. J Am Chem Soc, 2012, 134: 8094–8097
For determinations of the relative configurations of the diastereomers, see the Supporting Information online
Xu J, Mou C, Zhu T, Song BA, Chi YR. Org Lett, 2014, 16: 3272–3275
Li Z, Li X, Cheng JP. J Org Chem, 2017, 82: 9675–9681
Niu Y, Wang N, Muñoz A, Xu J, Zeng H, Rovis T, Lee JK. J Am Chem Soc, 2017, 139: 14917–14930
Wang Z, Wang F, Xue XS, Ji P. Org Lett, 2018, 20: 6041–6045
Huang R, Chen X, Mou C, Luo G, Li Y, Li X, Xue W, Jin Z, Chi YR. Org Lett, 2019, 21: 4340–4344
Smith MK, Tunge JA. Org Lett, 2017, 19: 5497–5500
Mao PF, Zhou LJ, Zheng AQ, Miao CB, Yang HT. Org Lett, 2019, 21: 3153–3157
Gómez JE, Guo W, Gaspa S, Kleij AW. Angew Chem Int Ed, 2017, 56: 15035–15038
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21772029, 21801051, 21961006, 22071036, 82360589, 81360589), The 10 Talent Plan (Shicengci) of Guizhou Province ([2016]5649), the Guizhou Province Returned Oversea Student Science and Technology Activity Program [(2014)-2], the Science and Technology Department of Guizhou Province ([2018]2802, [2019]1020), the Program of Introducing Talents of Discipline to Universities of China (111 Program, D20023) at Guizhou University, Frontiers Science Center for Asymmetric Synthesis and Medicinal Molecules, Department of Education, Guizhou Province [Qianjiaohe KY (2020)004], the Guizhou Province First-Class Disciplines Project [(Yiliu Xueke Jianshe Xiangmu)-GNYL(2017)008], Guizhou University of Traditional Chinese Medicine (China), and Guizhou University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest The authors declare no conflict of interest.
Supporting Information
11426_2021_9989_MOESM1_ESM.pdf
Carbene-Catalyzed Activation of Cyclopropylcarbaldehydes for Mannich Reaction and δ-Lactam Formation: Remote Enantioselecitvity Control and Dynamic Kinetic Asymmetric Transformation
Rights and permissions
About this article
Cite this article
Lv, J., Xu, J., Pan, X. et al. Carbene-catalyzed activation of cyclopropylcarbaldehydes for mannich reaction and δ-lactam formation: remote enantioselecitvity control and dynamic kinetic asymmetric transformation. Sci. China Chem. 64, 985–990 (2021). https://doi.org/10.1007/s11426-021-9989-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-021-9989-1