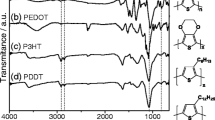
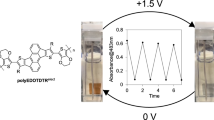
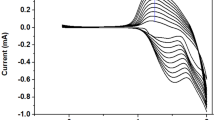
Abstract
Thieno[3,2-b]thiophene (TT) monomers end-capped with 3,4-ethylenedioxythiophene (EDOT) moieties are electropolymerized to form π-conjugated polymers with distinct electrochromic (EC) properties. Steric and electronic factors (electron donor and acceptor substituents) in the side groups of the TT core, as well as the structure of the polymer backbone strongly affect the electrochemical and optical properties of the polymers and their electrochromic characteristics. The studied polymers show low oxidation potentials, tunable from–0.78 to +0.30 V (vs. Fc/Fc+) and the band gaps from 1.46 to 1.92 eV and demonstrate wide variety of color palettes in polymer films in different states, finely tunable by structural variations in the polymer backbone and the side chains. EC materials of different colors in their doped/dedoped states have been developed (violet, deep blue, light blue, green, brown, purple-red, pinkish-red, orange-red, light gray, cyan and colorless transparent). High optical contrast (up to 79%), short response time (0.57–0.80 s), good cycling stability (up to 91% at 2000 cycles) and high coloration efficiency (up to 234.6 cm2 C–1) have been demonstrated and the influence of different factors on the above parameters of EC polymers have been discussed.
Similar content being viewed by others
References
Dyer AL, Österholm AM, Shen DE, Johnson KE, Reynolds JR. Conjugated electrochromic polymers: structure-driven colour and processing control. In: Mortimer RJ, Rosseinsky DR, Monk PMS, Eds. Electrochromic Materials and Devices. Part I ElectrochromicMaterials and Processing. Weinheim: Wiley-VCH, 2015. 113–169
Platt JR. J Chem Phys, 1961, 34: 862–863
Mortimer RJ. Chem Soc Rev, 1997, 26: 147
Yeh MH, Lin L, Yang PK, Wang ZL. ACS Nano, 2015, 9: 4757–4765
Runnerstrom EL, Llordés A, Lounis SD, Milliron DJ. Chem Commun, 2014, 50: 10555–10572
Granqvist CG, Azens A, Isidorsson J, Kharrazi M, Kullman L, Lindström T, Niklasson GA, Ribbing CG, Rönnow D, Strømme Mattsson M, Veszelei M. J Non-Crystalline Solids, 1997, 218: 273–279
Österholm AM, Shen DE, Kerszulis JA, Bulloch RH, Kuepfert M, Dyer AL, Reynolds JR. ACS Appl Mater Interfaces, 2015, 7: 1413–1421
Beaujuge PM, Reynolds JR. Chem Rev, 2010, 110: 268–320
Xu T, Walter EC, Agrawal A, Bohn C, Velmurugan J, Zhu W, Lezec HJ, Talin AA. Nat Commun, 2016, 7: 10479
Sonmez G, Sonmez HB. J Mater Chem, 2006, 16: 2473–2477
Invernale MA, Ding Y, Sotzing GA. ACS Appl Mater Interfaces, 2010, 2: 296–300
Yu H, Shao S, Yan L, Meng H, He Y, Yao C, Xu P, Zhang X, Hu W, Huang W. J Mater Chem C, 2016, 4: 2269–2273
Chandrasekhar P, Zay BJ, Birur GC, Rawal S, Pierson EA, Kauder L, Swanson T. Adv Funct Mater, 2002, 12: 95–103
Chandrasekhar P, Zay BJ, McQueeney T, Scara A, Ross D, Birur GC, Haapanen S, Kauder L, Swanson T, Douglas D. Synth Met, 2003, 135-136: 23–24
Abidin T, Zhang Q, Wang KL, Liaw DJ. Polymer, 2014, 55: 5293–5304
Beverina L, Pagani GA, Sassi M. Chem Commun, 2014, 50: 5413–5430
Amb CM, Dyer AL, Reynolds JR. Chem Mater, 2011, 23: 397–415
Bulloch RH, Kerszulis JA, Dyer AL, Reynolds JR. ACS Appl Mater Interfaces, 2015, 7: 1406–1412
Kerszulis JA, Amb CM, Dyer AL, Reynolds JR. Macromolecules, 2014, 47: 5462–5469
Bulloch RH, Reynolds JR. J Mater Chem C, 2016, 4: 603–610
Kirchmeyer S, Reuter K. J Mater Chem, 2005, 15: 2077–2088
Jonas F, Schrader L. Synth Met, 1991, 41: 831–836
Heywang G, Jonas F. Adv Mater, 1992, 4: 116–118
Dietrich M, Heinze J, Heywang G, Jonas F. J Electroanal Chem, 1994, 369: 87–92
Xia Y, Ouyang J. ACS Appl Mater Interfaces, 2012, 4: 4131–4140
Kim YH, Sachse C, Machala ML, May C, Müller-Meskamp L, Leo K. Adv Funct Mater, 2011, 21: 1076–1081
Heuer HW, Wehrmann R, Kirchmeyer S. Adv Funct Mater, 2002, 12: 89–94
Invernale MA, Bokria JG, Ombaba M, Lee KR, Mamangun DMD, Sotzing GA. Polymer, 2010, 51: 378–382
Groenendaal L, Zotti G, Aubert PH, Waybright SM, Reynolds JR. Adv Mater, 2003, 15: 855–879
Groenendaal L, Jonas F, Freitag D, Pielartzik H, Reynolds JR. Adv Mater, 2000, 12: 481–494
Roncali J, Blanchard P, Frère P. J Mater Chem, 2005, 15: 1589–1610
Beaujuge PM, Amb CM, Reynolds JR. Acc Chem Res, 2010, 43: 1396–1407
Cinar ME, Ozturk T. Chem Rev, 2015, 115: 3036–3140
Skabara PJ. Fused oligothiophenes. In: Perepichka IF, Perepichka DF, Eds. Handbook of Thiophene-Based Materials: Applications in Organic Electronics and Photonics. Volume 1: Synthesis and Theory. New York: John Wiley & Sons, 2009. 219–254
Zhang Q, Wang Y, Kan B, Wan X, Liu F, Ni W, Feng H, Russell TP, Chen Y. Chem Commun, 2015, 51: 15268–15271
Tang W, Ke L, Tan L, Lin T, Kietzke T, Chen ZK. Macromolecules, 2007, 40: 6164–6171
Perepichka IF, Perepichka DF, Meng H, Wudl F. Adv Mater, 2005, 17: 2281–2305
Hamaguchi A, Negishi T, Kimura Y, Ikeda Y, Takimiya K, Bisri SZ, Iwasa Y, Shiro T. Adv Mater, 2015, 27: 6606–6611
Toksabay S, Hacioglu SO, Unlu NA, Cirpan A, Toppare L. Polymer, 2014, 55: 3093–3099
Turbiez M, Frère P, Leriche P, Mercier N, Roncali J. Chem Commun, 2005, 1161–1163
Capan A, Ozturk T. Synth Met, 2014, 188: 100–103
Shi J, Zhu X, Xu P, Zhu M, Guo Y, He Y, Hu Z, Murtaza I, Yu H, Yan L, Goto O, Meng H. Macromol Rapid Commun, 2016, 37: 1344–1351
Xu P, Murtaza I, Shi J, Zhu M, He Y, Yu H, Goto O, Meng H. Polym Chem, 2016, 7: 5351–5356
Zhu X, Zhu Y, Murtaza I, Shi J, He Y, Xu P, Zhu M, Goto O, Meng H. RSC Adv, 2016, 6: 75522–75529
Raimundo JM, Blanchard P, Frère P, Mercier N, Ledoux-Rak I, Hierle R, Roncali J. Tetrahedron Lett, 2001, 42: 1507–1510
Turbiez M, Frère P, Roncali J. J Org Chem, 2003, 68: 5357–5360
Turbiez M, Frère P, Allain M, Videlot C, Ackermann J, Roncali J. Chem Eur J, 2005, 11: 3742–3752
Turbiez M, Hergué N, Leriche P, Frère P. Tetrahedron Lett, 2009, 50: 7148–7151
Us CN, Icli Ozkut M. Macromolecules, 2016, 49: 3009–3015
Balan A, Baran D, Gunbas G, Durmus A, Ozyurt F, Toppare L. Chem Commun, 2009, 6768
Amb CM, Beaujuge PM, Reynolds JR. Adv Mater, 2010, 22: 724–728
Wang JL, Yin QR, Miao JS, Wu Z, Chang ZF, Cao Y, Zhang RB, Wang JY, Wu HB, Cao Y. Adv Funct Mater, 2015, 25: 3514–3523
Ledwon P, Zassowski P, Jarosz T, Lapkowski M, Wagner P, Cherpak V, Stakhira P. J Mater Chem C, 2016, 4: 2219–2227
Gu PY, Zhang J, Long G, Wang Z, Zhang Q. J Mater Chem C, 2016, 4: 3809–3814
Shi P, Amb CM, Dyer AL, Reynolds JR. ACS Appl Mater Interfaces, 2012, 4: 6512–6521
Gaupp CL, Welsh DM, Rauh RD, Reynolds JR. Chem Mater, 2002, 14: 3964–3970
Liang L, Zhang J, Zhou Y, Xie J, Zhang X, Guan M, Pan B, Xie Y. Sci Rep, 2013, 3: 1936
Yao DD, Rani RA, O’Mullane AP, Kalantar-zadeh K, Ou JZ. J Phys Chem C, 2014, 118: 10867–10873
Ye Q, Neo WT, Lin T, Song J, Yan H, Zhou H, Shah KW, Chua SJ, Xu J. Polym Chem, 2015, 6: 1487–1494
Neo WT, Ye Q, Lin TT, Chua SJ, Xu J. Sol Energ Mat Sol C, 2015, 136: 92–99
Tehrani P, Hennerdal LO, Dyer AL, Reynolds JR, Berggren M. J Mater Chem, 2009, 19: 1799–1802
Huang JH, Hsu CY, Hu CW, Chu CW, Ho KC. ACS Appl Mater Interfaces, 2010, 2: 351–359
Perepichka IF, Besbes M, Levillain E, Sallé M, Roncali J. Chem Mater, 2002, 14: 449–457
Acknowledgments
This work was supported by Shenzhen Key Laboratory of Organic Optoelectromagnetic Functional Materials of Shenzhen Science and Technology Plan (ZDSYS20140509094114164), the Shenzhen Peacock Program (KQTD2014062714543296), Shenzhen Science and Technology Research Grant (JCYJ20140509093817690), Nanshan Innovation Agency Grant (KC2015ZDYF0016A), Guangdong Key Research Project (2014B090914003, 2015B090914002), Guangdong Talents Project, the National Basic Research Program of China (2015CB856505), the National Natural Science Foundation of China (51373075), Guangdong Academician Workstation (2013B090400016), the Natural Science Foundation of Guangdong Province (2014A030313800). Igor F. Perepichka thanks to the Santander Universities Research Mobility Award to support his travel to Shenzhen.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, M., Li, W., Xu, P. et al. Molecular engineering tuning optoelectronic properties of thieno[3,2-b]thiophenes-based electrochromic polymers. Sci. China Chem. 60, 63–76 (2017). https://doi.org/10.1007/s11426-016-0305-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-016-0305-9