Abstract
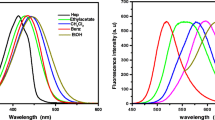
Six kinds of terbium ternary complexes with halo-benzoic acids were synthesized. Their compositions were determined by C, H elemental analyzer and EDTA titration. The infrared spectra, ultraviolet absorption spectra, and fluorescence spectra were also measured to identify the complexes. Elemental analysis showed that the compositions of these complexes were Tb(p-BrBA)3·H2O, Tb(p-ClBA)3·2H2O, Tb(p-FBA)3·H2O, Tb(o-FBA)3·2H2O, Tb(o-ClBA)3·H2O, and Tb(o-BrBA)3·H2O, respectively. The monodispersed Ag@SiO2 core-shell nanoparticles with silica thicknesses of 10, 15, and 25 nm were successfully prepared and characterized by transmission-electron microscopy. Fluorescence intensities of the complexes were detected before and after Ag@SiO2 core-shell nanoparticles were added; the enhancement times were related to the silica-shell thickness. The fluorescence enhancement times were largest when the thickness of the silica shell was 25 nm. The mechanism may be attributed to the localized surface-plasmon resonance. Furthermore, the enhancement effect of terbium fluoro-benzoate complexes was the strongest in these complexes. This result may be attributed to the hydrogen bond between the hydroxyl on the surface of the silica shell and the fluorine atom.
Similar content being viewed by others
References
Jami AK, Baskar V, Sanudo EC. New structural form of a tetranuclear lanthanide hydroxo cluster: Dy4 analogue display slow magnetic relaxation. Inorg Chem, 2013, 52: 2432–2438
Kido J, Okamoto Y. Organo lanthanide metal complexes for electroluminescent materials. Chem Rev, 2002, 102: 2357–2368
Ma DY, Wang WX, Li YW, Li J, Daiguebonne C, Calvez G, Guillou O. In situ 2,5-pyrazinedicarboxylate and oxalate ligands synthesis leading to a microporous europium-organic framework capable of selective sensing of small molecules. Cryst Eng Comm, 2010, 12: 4372–4379
Liu GX, Zhou H, Ren XM. Synthesis, structure and near-infrared luminescence of a new 2D praseodymium(III) coordination polymer. J Rare Earth, 2011, 29: 1100–1104
Tsukube H, Juanes S. Lanthanide complexes in molecular recognition and chirality sensing of biological substrates. Chem Rev, 2002, 102: 2389–2403
Galdwell JP, Henderson W, Kim ND. Luminescent visualization of latent fingerprints by direct reaction with a lanthanide shift reagent. J Forensic Sci, 2001, 46: 1332–1341
Dinca M, Yu AF, Long JR. Microporous metal-organic frameworks incorporating 1,4-benzeneditetrazolate: syntheses, structures, and hydrogen storage properties. J Am Chem Soc, 2006, 128: 8904–8913
Gusev AN, Hasegawa M, Shimizu T, Fukawa T, Sakurai S, Nishchymenko GA, Shul’gin VF, Meshkova SB, Linert W. Synthesis, structure and luminescence studies of Eu(III), Tb(III), Sm(III), Dy(III) cationic complexes with acetylacetone and bis(5-(pyridine-2-yl)-1,2, 4-triazol-3-yl)propane. Inorg Chim Acta, 2013, 406: 279–284
An BL, Cheah KW, Wong WK, Shi JX, Xu NS, Yang YS, Gong ML. Synthesis and luminescence of a novel conjugated europium complex with 6-paramethylaniline carbonyl 2-pyridine carboxylate. J Alloys Compd, 2003, 352: 143–147
Zhou ZC. The Synthesis, Fluorescence Properties and Theoretical Research of Photoluminescence of Rare Earth (Eu, Tb) Complexes. Doctor Dissertation. Changsha: Central South University, 2002
Franville AC, Mahiou R, Zambon D, Cousseins JC. Molecular design of luminescent organic-inorganic hybrid materials activated by europium (III) ions. Solid State Sci, 2001, 3: 211–222
Ding YF, Yu XB, Xiong J, Wang ZM, Wang WM, Huang Y. Preparation of benzoic europium and benzoic terbium phosphors by solid-state reaction at room temperature. Chin Rare Earth, 2003, 24: 18–21
Lis S, Hnatejko Z, But S, Szyczewsk A, Elbanowski M. Spectroscopic studies of the lanthanide(III) ions with pyridine carboxylic acid N-oxide ligands and in mixed ligand complexes. Mol Phys, 2003, 101: 977–981
Zheng XJ, Jin LP, Wang ZM, Yan CH, Lu SZ, Li Q. Structure and photophysical properties of europium complexes of succinamic acid and 1,10-phenanthroline. Polyhedron, 2003, 22: 323–330
Aslan K, Gryczynski I, Malicka J, Matveeva E, Lakowicz JR, Geddes CD. Metal-enhanced fluorescence: an emerging tool in biotechnology. Curr Opin Biotechnol, 2005, 16: 55–62
Lakowicz JR, Ray K, Chowdhury M, Szmacinski H, Fu Y, Zhang J, Nowaczyk K. Plasmon-controlled fluorescence: a new paradigm in fluorescence spectroscopy. Analyst, 2008, 133: 1308–1346
Deng W, Goldys EM. Plasmonic approach to enhanced fluorescence for applications in biotechnology and the life sciences. Langmuir, 2012, 28: 10152–10163
Song YJ, Yanga WT, King M. Shape controlled synthesis of sub-3 nm Ag nanoparticles and their localized surface plasmonic properties. Chem Phys Lett, 2008, 455: 218–224
Jain PK, El-Sayed MA. Plasmonic coupling in noble metal nanostructures. Chem Phys Lett, 2010, 487: 153–164
Wang ZH, Liang QL, Wang YM, Luo GA. Carbon nanotube-intercalated graphite electrodes for simultaneous determination of dopamine and serotonin in the presence of ascorbic acid. J Electroanal Chem, 2003, 540: 129–134
Raj CR, Okajima T, Ohsaka T. Gold nanoparticle arrays for the voltammetric sensing of dopamine. J Electroanal Chem, 2003, 543: 127–133
Zhao YF, Zhao YL, Bai F. Synthesis, characterization and fluorescence properties of rare earth complexes RE(TPTZ)Cl3. Spectrosc Spect Anal, 2009, 29: 1929–1932
Tovmachenko OG, Graf C, van den Heuvel DJ, van Blaaderen A, Gerritsen HC. Fluorescence enhancement by metal-core/silica-shell nanoparticles. Adv Mater, 2006, 18: 91–95
Aslan K, Wu M, Lakowicz JR, Geddes CD. Fluorescent core-shell Ag@SiO2 nanocomposites for metal-enhanced fluorescence and single nanoparticle sensing platforms. J Am Chem Soc, 2007, 129: 1524–1525
Liu SH, Zhang ZH, Han MY. Gram-scale synthesis and biofunctionalization of silica-coated silver nanoparticles for fast colorimetric DNA detection. Anal Chem, 2005, 77: 2595–2600
Graf C, Vossen DLJ, Imhof A, van Blaaderen A. A general method to coat colloidal particles with silica. Langmuir, 2003, 19: 6693–6700
Zhu RH, Kok WT. Determination of catecholamines and related compounds by capillary electrophoresis with postcolumn terbium complexation and sensitized luminescence detection. Anal Chem, 1997, 69: 4010–4016
Gryczynski I, Malika J, Gryczynski Z, Geddes CD, Lakowicz JR. The CFS engineers the intrinsic radiative decay rate of low quantum yield fluorophores. J Fluoresc, 2002, 12: 11–13
Guo LQ, Guan AH, Lin XL, Zhang CL, Chen GN. Preparation of a new core-shell Ag@SiO2 nanocomposite and its application for fluorescence enhancement. Talanta, 2010, 82: 1696–1700
Geddes CD, Lakowicz JR. Metal-enhanced fluorescence. J Fluoresc, 2002, 12: 121–129
Chu FH, Zhan YG, Yang JJ, Wang JY. Using Au/SiO2 core-shell structure to enhance the fluorescence of MEH-PPV in the detection of nitrated aromatic explosives. Optik, 2013, 124: 1338–1341
Maliwal BP, Malicka J, Gryczynski I, Gryczynski Z, Lakowicz JR. Fluorescence properties of labeled proteins near silver colloid surfaces. Biopolymers, 2003, 70: 585–594
Malicka J, Gryczynski I, Maliwal BP, Fang JY, Lakowicz JR. Fluorescence spectral properties of cyanine dye labeled DNA near metallic silver particles. Biopolymers, 2003, 72: 96–104
Parfenov A, Gryczynski I, Malicka J, Geddes CD, Lakowicz JR. Enhanced fluorescence from fluorophores on fractal silver surfaces. J Phys Chem B, 2003, 107: 8829–8833
Gryczynski I, Malicka J, Holder E, DiCesare N, Lakowicz JR. Effects of metallic silver particles on the emission properties of [Ru(bpy)3]2+. Chem Phys Lett, 2003, 372: 409–414
Zhang SW, Yu AX, Liu SL, Jiang JQ, Liu XY. Morphology and properties of UV curable WPU/SiO2 nanocomposites. Polym Mater Sci Eng, 2011, 27: 101–105
Liu JH, Wang Q, Yu M, Li SM, Zhan ZW, Zhang J, Wang M. Effect of silica nanoparticles on performance of sol-gel coatings. Chin J Inorg Chem, 2012, 28: 873–880
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhou, D., Lin, X., Wang, A. et al. Fluorescence enhancement of Tb3+ complexes by adding silica-coated silver nanoparticles. Sci. China Chem. 58, 979–985 (2015). https://doi.org/10.1007/s11426-014-5265-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-014-5265-x