Abstract
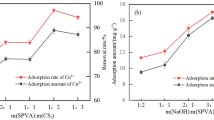
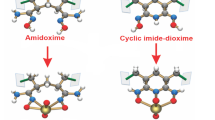
Amidoxime-based adsorbents are widely studied as the main adsorbent in the recovery of uranium from seawater. However, the adsorption rate and loading capacity of such adsorbents should be further improved due to the economic viability consideration. In this paper, polyvinyl alcohol functionalized with amidoxime (PVA-g-AO) has been prepared as a new adsorbent for uranium (VI) adsorption from aqueous solution. The physicochemical properties of PVA-g-AO were investigated using infrared spectroscopy (IR), scanning electron microscope (SEM), X-ray diffraction (XRD), and X-ray photoelectron spectroscopy (XPS). Results showed that the ligand monomers were successfully grafted onto the matrixes. The XRD and XPS analysis showed that uranium was adsorbed in metal ionic form rather than in crystal form. Uranyl (U (VI)) adsorption properties onto PVA-g-AO were evaluated. The adsorption of U (VI) by PVA-g-AO was fast, with an equilibrium time of less than 50 min. Additionally the maximum adsorption capacity reached 42.84 mg/g at pH 4.0.
Similar content being viewed by others
References
Davies RV, Dr Kennedy J, Mcilroy RW, Dr Spence R. Extraction of uranium from sea water. Nature, 1964, 203: 1110–1115
Rao LF. Recent International R&D Activities in the Extraction of Uranium from Seawater. Lawrence Berkeley National Laboratory. 2011
Tamada M. Current Status of Technology for Collection of Uranium from Seawater. Japan Atomic Energy Agency, 2009
Gorden AEV, Xu JD, Raymond, KN. Rational design of sequestering agents for plutonium and other actinides. Chem Rev, 2003, 103: 4207–4282
Xu J, Raymond KN. Uranyl sequestering agents: Correlation of properties and efficacy with structure for UO2 2+ complexes of linear tetradentate 1-methyl-3-hydroxy-2(1H)-pyridinone ligands. Inorg Chem, 1999, 38: 308–315
Sather AC, Berryman OB, Rebek J Jr. Selective recognition and extraction of the uranyl ion. J Am Chem Soc, 2010, 132: 13572–13574
Beer S, Berryman OB, Ajami D. Encapsulation of the uranyl dication. Chem Sci, 2010, 1: 43–47
Yang JB, Volesky B. Modeling uranium-proton ion exchange in biosorption. Enviro Sci Technol, 1999, 33: 4079–4082
Koide Y, Terasaki H, Sato H, Shosenji H, Yamada K. Flotation of uranium from seawater with phosphate esters of C-undecylcalix[4] resorcinarene. Bull Chem Soc Jpn, 1996, 69: 785–790
Rivas BL, Maturana HA, Villegas S. Adsorption behavior of metal ions by amidoxime chelating resin. J Appl Polym Sci, 2000, 77: 1994–1999
Pekel N, Şahiner N, Guven. O. Use of amidoximated acrylonitrile/N-vinyl 2-pyrrolidone interpenetrating polymer networks for uranyl ion adsorption from aqueous systems. J Appl Polym Sci, 2001, 81: 2324–2329
Ozmen F, Kavakli PA. Removal of phosphate by using copper-loaded poly (N-vinylimidazole) hydrogels as polymeric ligand exchanger. J. Appl Polym Sci, 2011, 119: 613–619
Çaykara T, Alaslan ŞŞ, İnam R. Competitive adsorption of uranyl ions in the presence of Pb (II) and Cd (II) ions by poly (glycidyl methacrylate) microbeads carrying amidoxime groups and polarographic determination. J Appl Polym Sci, 2007, 104: 4168–4172
Çaykara T, Alaslan ŞŞ. Preparation and characterization of novel poly (glycidyl methacrylate) beads carrying amidoxime groups. J Appl Polym Sci, 2007, 106: 2126–2131
Egawa H, Nakayama M, Nonaaka T, Yamamoto H, Uemura K. Recovery of uranium from seawater. V. Preparation and properties of the macroreticular chelating resins containing amidoxime and other functional groups. J Appl Polym Sci, 1987, 34: 1557–1575
Egawa H, Kabay N, Shuto T, Jyo A. Recovery of uranium from sea water. XII. Preparation and characterization of lightly crosslinked highly porous chelating resins containing amidoxime groups. J Appl Polym Sci, 1992, 46: 129–142
Egawa H, Nonaka T, Abe S, Nakayama M. Recovery of uranium from seawater. X. Pore structure and uranium adsorption of macroreticular chelating resin containing amidoxime groups. J Appl Polym Sci, 1992, 45: 837–841
Kavaklı P A, Güven O. Removal of concentrated heavy metal ions from aqueous solutions using polymers with enriched amidoxime groups. J Appl Polym Sci, 2004, 93: 1705–1710
Çaykara T, Alaslan ŞŞ, Gürü M, Bodugöz H, Güvenc O. Preparation and characterization of poly(isobutyl methacrylate)microbeads with grafted amidoxime groups. Radiat Phys Chem, 2007, 76: 1569–1576
Atta AM, Sayed SA, Farag AB. Ismail HS. Application of crosslinked acrylamidoxime/2-acrylamido-2-methylpropane sulfonic acid copolymer in wastewater treatment. J Dispersion Sci Tech, 2011, 32: 1285–1295
Seko N, Ninh NTY, Tamada M. Elusion grafting of glycidyl methacrylate onto polyethylene fiber. Radiat Phys Chem, 2010, 79: 22–26
Yao ZH, Rao L, Xu J. Synthesis of a new type of adsorbent containing carboxyl and amidoxime group by preirradiation grafting and its absorption of metal ions. J Appl Polym Sci, 2002, 83: 1986–1992
Chi FT, Xiong J, Hou JW, Gu M, Hu S, Wang XL. Improvement in uranium adsorption properties of amidoxime-based adsorbent through cografting of amine group. J Disper Sci Tech, 2013, 34: 1–7
Bonato M, Allen GC, Scott TB. Reduction of U (VI) to U (IV) on the surface of TiO2 anatase nanotubes. Micro Nano Lett, 2008, 3: 57–61
Scott TB, Allen GC, Heard PJ, Randell M. Reduction of U(VI) to U(IV) on the surface of magnetite. Geoch Cosmoch Acta, 2005, 69: 5639–5643
Yuan LY, Liu YL, Shi WQ, Lv YL, Lan JH, Zhao YL, Chai ZF. High performance of phosphonate-functionalized mesoporous silica for U (VI) sorption from aqueous solution. Dalton Trans, 2011, 40: 7446–7453
Lagergren S, Zur theorie der sogenannten adsorption geloester stoffe. Kungliga Svenska Vetenskapsakad, Handl, 1898, 24: 1–39
Ho YS, McKay G. Pseudo-second order model for sorption processes. Process Biochem, 1999, 34: 451–465
Boparai HK, Joseph MD, O’Carroll M. Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zerovalent iron particles. J Hazard Mater, 2011, 186: 458–465
Pillewan P, Mukherjee S, Roychowdhury T, Das S, Bansiwal A, Rayalu S. Removal of As(III) and As(V) from water by copper oxide incorporated mesoporous alumina. J Hazard Mater, 2011, 186: 367–375
Freundlich H, Über die adsorption in lösungen (adsorption in solution). Z Phys Chem, 1906, 57: 384–470
Temkin MJ, Pyzhev V. Recent modifications to Langmuir isotherms. Acta Physiochim URSS, 1940, 12: 217–222
Metilda P, Sanghamitra K, Gladis JM, Naidu GRK, Rao TP. Amberlite XAD-4 functionalized with succinic acid for the solid phase extractive preconcentration and separation of uranium (VI), Tanlata, 2005, 65: 192–200
Someda HH, Sheha RR. Solid phase extractive preconcentration of some actinide elements using impregnated carbon. Radiochem, 2008, 50: 50–56
Starvin AM, Rao TP. Solid phase extractive preconcentration of uranium (VI) onto diarylazobisphenol modified activated carbon. Talanta, 2004, 63: 225–232
Merdivan M, Düz MZ, Hamamci C. Sorption behavior of uranium (VI) with N,N-dibutyl-N′-benzoylthiourea impregnated in Amberlite XAD-16. Talanta, 2001, 55: 639–645
Merdivan M, Seyhan S, Gok C. Use of benzoylthiourea immobilized on silica gel for separation and preconcentration of uranium (VI). Microch Acta, 2006, 154: 109–114
Kim JH, Lee HI, Yeon JW, Jung YJ, Kim JM. Removal of uranium (VI) from aqueous solutions by nanoporous carbon and its chelating polymer composite. J Radioanal Nucl Chem, 2010, 286: 129–133
Venkatesan KA, Sukumaran V, Antony MP, Rao PRV. Extraction of uranium by amine, amide and benzamide grafted covalently on silica gel. J Radioanal Nucl Chem, 2010, 260: 443–450
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chi, F., Hu, S., Xiong, J. et al. Adsorption behavior of uranium on polyvinyl alcohol-g-amidoxime: Physicochemical properties, kinetic and thermodynamic aspects. Sci. China Chem. 56, 1495–1503 (2013). https://doi.org/10.1007/s11426-013-5003-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-013-5003-9