Abstract
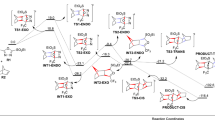
A domino [4 + 2]/retro [4 + 2] cycloaddition process of cyclohexadienes with arylethynes or benzyne providing access to biaryls and polycyclic aromatics has been studied theoretically using density functional theory calculations. It has been found that the initial Diels-Alder (D-A) reaction acts as the rate-determining step and the consequent [4 + 2] cycloreversion reaction is feasible under the conditions used. Furthermore, the D-A reaction affects the regioselectivity, the origin of which is essentially derived from the good match of orbital coefficients between dienes and dienophiles as shown by using frontier molecular orbital (FMO) theory. Further investigation of the reactivity reveals that the reactions are predicted to fail to occur if an electron-donor group in the diene or an electron-acceptor group in the dienophile is lacking, as a consequence of the increased FMO energy gap. By further exploring the scope of substrates computationally, benzyne as an active dienophile was predicted to react with a variety of dienes in a cascade reaction under mild conditions with a low energy barrier, with the rate-determining step being the retro [4 + 2] cycloaddition.
Similar content being viewed by others
References
Diels O, Alder K. Synthesen in der hydroaromatischen Reihe. Liebigs Ann Chem, 1928, 460: 98–122
Takao KI, Munakata R, Tadano KI. Recent advances in natural product synthesis by using intramolecular Diels-Alder reactions. Chem Rev, 2005, 105: 4779–4807
Reed JA, Schilling CL, Tarvin RF, Rettig TA, Stille JK. Diels-Alder reactions of 2-pyrones. Direction of the addition reaction with acetylenes. J Org Chem, 1969, 34: 2188–2192
McDonald E, Suksamrarn A, Wylie RD. Diels-Alder reactivity of oxygenated dienes and furans. Synthesis of oxygenated biphenyls. J Chem Soc, Perkin Trans 1, 1979: 1893–1900
Weinreb SM, Basha FZ, Hibino S, Khatri NA, Kim D, Pye WE, Wu TT. Total synthesis of the antitumor antibiotic streptonigrin. J Am Chem Soc, 1982, 104: 536–544
Boger DL, Panek JS, Duff SR. Inverse electron demand Diels-Alder reactions of heterocyclic azadienes: Formal total synthesis of streptonigrin. J Am Chem Soc, 1985, 107: 5745–5754
Effenberger F, Ziegler T. Diels-Alder-reaktionen mit 2H-pyran-2-onen: Reaktivität und selektivität. Chem Ber, 1987, 120: 1339–1346
Sain B, Sandhu JS. A facile one-pot synthesis of unsymmetrical biaryl-2-carbonitriles by novel reaction of ylidenemalononitriles with dienamines. J Org Chem, 1990, 55: 2545–2546
Royles BJL, Smith DM. The ‘inverse electron-demand’ Diels-Alder reaction in polymer synthesis. Part 1. A convenient synthetic route to diethynyl aromatic compounds. J Chem Soc, Perkin Trans, 1994: 355–358
D’Auria M. Regioselective photochemical Diels-Alder reaction on thiophene derivatives. Tetrahedron Lett, 1995, 36: 6567–6570
Avenoza A, Busto JH, Cativiela C, Peregrina JM. New efficient synthesis of 4-amino-3-arylphenols. Synthesis, 1995: 671–674
Balzs L, Kdas I, Tõke L. Inverse-electron-demand Diels-Alder reactions of 4-aryl-2-pyrones with electron-rich dienophiles. Tetrahedron Lett, 2000, 41: 7583–7587
Watson MD, Fechtenkotter A, Mullen K. Big is beautiful—“aromaticity” revisited from the viewpoint of macromolecular and supramolecular benzene chemistry. Chem Rev, 2001, 101: 1267–1300
Min SH, Kim YW, Choi S, Park KB, Cho CG. Expedient syntheses of unsymmetrical 4-bromo-2-carboxyl-biaryls via Diels-Alder cycloadditions of 3, 5-dibromo-2-pyrone with vinyl arenes, followed by one pot, three step aromatization reactions. Bull Korean Chem Soc, 2002, 23: 1021–1022
Hilt G, Smolko KI. Cobalt(I)-catalyzed neutral Diels-Alder reactions of 1, 3-diynes with acyclic 1,3-dienes. Synthesis, 2002: 686–692
Hilt G, Smolko KI, Lotsch BV. Cobalt (I)-catalyzed neutral Diels-Alder reactions of oxygen-functionalized acyclic 1,3-dienes with alkynes. Synlett, 2002: 1081–1084
Moore JE, York M, Harrity JPA. A metal-free cycloaddition approach to highly substituted aromatic boronic esters. Synlett, 2005: 860–862
Yamato T, Miyamoto M, Hironaka T, Miura Y. Synthesis and Diels-Alder reactions of 1,2-dimethylene [2.n] metacyclophanes. Org Lett, 2005, 7: 3–6
Hilt G, Galbiati F, Harms K. A modular approach for the synthesis of dibenzoazepine derivatives. Synthesis, 2006: 3575–3584
Ashburn BO, Carter RG. Diels-Alder approach to polysubstituted biaryls: Rapid entry to tri- and tetra-ortho-substituted phosphorus-containing biaryls. Angew Chem Int Ed, 2006, 45: 6737–6741
Ashburn BO, Carter RG, Zakharov LN. Synthesis of tetra-ortho-substituted, phosphorus-containing and carbonyl-containing biaryls utilizing a Diels-Alder approach. J Am Chem Soc, 2007, 129: 9109–9116
Naffziger MR, Ashburn BO, Perkins JR, Carter RG. Diels-Alder approach for the construction of halogenated, o-nitro biaryl templates and application to the total synthesis of the anti-HIV agent siamenol. J Org Chem, 2007, 72: 9857–9865
Ashburn BO, Carter RG. Diels-Alder approach to tetra-ortho-substi-tuted biaryls employing propargylic tertiary alcohols as dienophiles. J Org Chem, 2007, 72: 10220–10223
Ashburn BO, Carter RG. Diels-Alder approach to biaryls (DAB): Importance of the ortho-nitro moiety in the [4 + 2] cycloaddition. Org Biomol Chem, 2008, 6: 255–257
Ashburn BO, Rathbone LK, Camp EH, Carter RG. A Diels-Alder approach to biaryls (DAB): Synthesis of the western portion of TMC-95. Tetrahedron, 2008, 64: 856–865
McIntosh ML, Johnston RC, Pattawong O, Ashburn BO, Naffziger MR, Cheong PHY, Carter RG. Synthesis and computational analysis of densely functionalized triazoles using o-nitrophenylalkynes. J Org Chem, 2012, 77: 1102–1112
Abid O, Nawaz M, Ibad MF, Khera RA, Iaroshenkoa V, Langer P. Synthesis of functionalized arylpyridines and -pyrimidines by domino [4 + 2]/retro [4 + 2] cycloadditions of electron-rich dienes with alkynylpyridines and -pyrimidines. Org Biomol Chem, 2011, 9: 2185–2191
Hassan J, Sevignon M, Gozzi C, Schulz E, Lemaire M. Aryl-aryl bond formation one century after the discovery of the Ullmann reaction. Chem Rev, 2002, 102: 1359–1469
Shimizu H, Nagasaki I, Saito T. Recent advances in biaryl-type bisphosphine ligands. Tetrahedron, 2005, 61: 5405–5432
Bringmann G, Mortimer AJ, Keller PA, Gresser MJ, Garner J, Breuning M. Atroposelective synthesis of axially chiral biaryl compounds. Angew Chem Int Ed, 2005, 44: 5384–5427
Ladouceur GH, Cook JH, Doherty EM, Schoen WR, MacDougall ML, Livingston JN. Discovery of 5-hydroxyalkyl-4-phenylpyridines as a new class of glucagon receptor antagonists. Bioorg Med Chem Lett, 2002, 12: 461–464
Boger DL, Weinreb SM. Hetero Diels-Alder Methodology in Organic Synthesis. San Diego: Academic Press, 1987
Bodwell G, Li J. Concise synthesis and transannular inverse electron demand Diels-Alder reaction of [3](3,6)pyridazino[3](1,3)indolophane. Rapid access to a pentacyclic indoloid system. Org Lett, 2002, 4: 127–130
Soenen DR, Zimpleman JM, Boger DL. Synthesis and inverse electron demand Diels-Alder reactions of 3,6-bis(3,4-dimethoxybenzoyl)-1,2,4,5-tetrazine. J Org Chem, 2003, 68: 3593–3598
Yeung BKS, Boger DL. Synthesis of isochrysohermidin-distamycin hybrids. J Org Chem, 2003, 68: 5249–5253
Sadasivam DV, Prasad E, Flowers RA, Birney DM. Stopped-flow kinetics of tetrazine cycloadditions; experimental and computational studies toward sequential transition states. J Phys Chem A, 2006, 110: 1288–1294
Gomez-Bengoa E, Helm MD, Plant A, Harrity JPA. The participation of alkynylboronates in inverse electron demand [4 + 2] cycloadditions: A mechanistic study. J Am Chem Soc, 2007, 129: 2691–2699
Lodewyk MW, Kurth MJ, Tantillo DJ. Mechanisms for formation of diazocinones, pyridazines, and pyrazolines from tetrazines—Oxyanion-accelerated pericyclic cascades? J Org Chem, 2009, 74: 4804–4811
Kirk BH, Ess DH. Quantum mechanical inspection of the Diels-Alder approach to biaryls mechanism. Tetrahedron Lett, 2011, 52: 1245–1249
Becke AD. Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys, 1993, 98: 5648–5652
Lee C, Yang W, Parr RG. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B, 1988, 37: 785–789
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ. Gaussian 09, Revision B.01. Wallingford CT: Gaussian, Inc, 2010
Zhao Y, Truhlar DG. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Acc, 2008, 120: 215–241
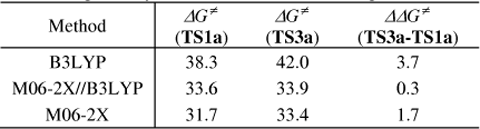
All the selected species shown in the following table were calculated with the same basis set 6-311++G(d,p) as B3LYP calculations. The computational results for TS1a and TS3a show that the free energy difference between TS1a and TS3a is 3.7 kcal mol-1 at the B3LYP level and 1.7 kcal mol-1 at the M06-2X level, both in good agreement with the experimental regioselectivity, although the free energies calculated at B3LYP are 6.6-8.6 kcal mol−1 higher than these at M06-2X. Alternatively, the combined B3LYP//M06-2X method gives poor regioselectivity. Thus, the calculations in the text were carried out primarily at the B3LYP//6-311++G(d,p) level of theory.
French AD, Kelterer AM, Johnson GP, Dowd MK, Cramer CJ. HF/6-31G* energy surfaces for disaccharide analogs. J Comput Chem, 2001, 22: 65–78
Hayden AE, Paton RS, Becker J, Lim YH, Nicolaou KC, Houk KN. Origins of regioselectivity of Diels-Alder reactions for the synthesis of bisanthraquinone antibiotic BE-43472B. J Org Chem, 2010, 75: 922–928 and references cited therein
Hammond GS. A correlation of reaction rates. J Am Chem Soc, 1955, 77: 334–338
Im GYJ, Bronner SM, Goetz AE, Paton RS, Cheong PHY, Houk KN, Garg NK. Indolyne experimental and computational studies: Synthetic applications and origins of selectivities of nucleophilic additions. J Am Chem Soc, 2010, 132: 17933–17944
Li XY, Xu JX. Theoretical insights into the metal-free and formal [2 + 2 + 2] cycloaddition strategy via intramolecular cascade propargylic ene/Diels-Alder reactions with tautomerization. Org Biomol Chem, 2011, 9: 5997–6003
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Li, X., Xu, J. Theoretical insights into the [4 + 2]/retro [4 + 2] cycloaddition approach to the synthesis of biaryls and polycyclic aromatics. Sci. China Chem. 56, 633–640 (2013). https://doi.org/10.1007/s11426-012-4821-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-012-4821-5