Abstract
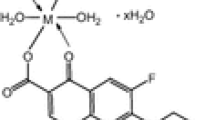
The reactions between gatifloxacin (GFX) and various one-electron oxidants, such as ·OH, N3 ·, Br2 ·−, and SO4 ·−, have been studied by pulse radiolysis techniques. The GFX radical anion formed in the reaction of GFX with eaq − could either be protonated or deprotonated, and the absorption of GFX radical anion was located at 390 nm. The transient species produced by the reaction of GFX with ·OH radical shows a broad band in the 380–600 nm region with a shoulder, while the oxidation by N3 ·, SO4 ·−, and Br2 ·− results in an absorption band with λ max = 370 nm. At neutral condition (pH 7), the rate constants of GFX reacting with ·OH, N3 ·, Br2 ·−, SO4 ·− and eaq − are estimated to be 1.0 × 1010, 3.1 × 109, 2.8 × 109, 3.0 × 109, and 1.8 × 1010 dm3 mol−1 s−1, respectively. From the pH dependence on the formation of electron adducts and on the rate constant of GFX with eaq −, the pKa of GFX radical anion is estimated to be 5.5 and 9.3.
Similar content being viewed by others
References
Domagala JM, Hann LD, Heifetz CL. New structure-activity relationships of the quinolone antibacterials using the target enzyme—The development and application of a DNA gyrase assay. J Med Chem, 1986, 29: 394–404
Wolfson JS, Hooper DC. Fluoroquinolone antimicrobial agents. Clin Microbiol Rev, 1989, 2: 378–424
Martinez L, Chignell CF. Photocleavage of DNA by the fluoroquinolone antibacterials. J Photoch Photobio B, 1998, 45: 51–59
Cheng, LL, Zhao P, Wang M, Zhu H, Zhu RR, Sun XY, Wang S. Photodamage and photooxidation mechanisms of bovine serum albumin. Acta Phys Chim Sin, 2009, 25: 25–29
Lhiaubet-Vallet V, Bosca F, Miranda MA. Photosensitized DNA damage: The case of fluoroquinolones. Photochem Photobiol, 2009, 85: 861–868
Fasani E, Mella M, Caccia D, Tassi S, Fagnoni, M, Albini A. The photochemistry of lomefloxacin — An aromatic carbene as the key intermediate in photodecomposition. Chem Commun, 1997, 28: 1329–1330
Martinez LJ, Li G, Chignell CF. Photogeneration of fluoride by the fluoroquinolone antimicrobial agents lomefloxacin and fleroxacin. Photochem Photobiol, 1997, 65: 599–602
Fasani E, Negra FFB, Mella M, Monti S, Albini A. Photoinduced C-F bond cleavage in some fluorinated 7-amino-4-quinolone-3-carboxylic acids. J Org Chem, 1999, 64: 5388–5395
Fasani E, Rampi M, Albini A. Photochemistry of some fluoroquinolones: Effect of pH and chloride ion. J Chem Soc, Perkin Trans 2, 1999, 1901–1907
Albini A, Monti S. Photophysics and photochemistry of fluoroquinolones. Chem Soc Rev, 2003, 32: 238–250
Cuquerella MC, Bosca F, Miranda MA. Photonucleophilic aromatic substitution of 6-fluoroquinolones in basic media: Triplet quenching by hydroxide anion. J Org Chem, 2004, 69: 7256–7261
Fasani E, Mella M, Albini A. Photochemistry of the phototoxic drug lomefloxacin: Paths observed in the presence of amines or NaOH and from the methyl ester. Eur J Org Chem, 2004, 24: 5075–5082
Cuquerella M., Miranda MA, Bosca F. Role of excited state intramolecular charge transfer in the photophysical properties of norfloxacin and its derivatives. J Phys Chem A, 2006, 110: 2607–2612
Cuquerella MC, Miranda MA, Bosca F. Generation of detectable singlet aryl cations by photodehalogenation of fluoroquinolones. J Phys Chem B, 2006, 110: 6441–6443
Fasani E, Monti S, Manet I, Tilocca F, Pretali L, Mella M, Albini A. Inter- and intramolecular photochemical reactions of fleroxacin. Org Lett, 2009, 11: 1875–1878
Vermeersch G, Ronfardharet JC, Bazin M, Carillet V, Morliere P, Santus R. Type-I and type-II photosensitization by the antibacterial drug nalixic acid—A laser flash-photolysis study. Photochem Photobiol, 1991, 54: 661–666
Mella M, Fasani E, Albini A, Photochemistry of 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(piperazin-1-yl)quinoline-3-carboxylic acid (= ciprofloxacin) in aqueous solutions. Helv Chim Acta, 2001, 84: 2508–2519
Preston SL, Drusano G. Gatifloxacin—A new fluoroquinolone for use in community-acquired pneumonia and other infections. Formulary, 1999, 34: 1002–1015
Perry CM, Balfour JAB, Lamb HM. Gatifloxacin. Drugs, 1999, 58: 683–696
Zhanel GG, Roberts D, Laing N, Nichol K, Wierzbowski A, Walkty A, Hoban DJ. Pharmacodynamic activity of respiratory fluoroquinolones (FQ) versus ciprofloxacin-resistant Streptococcus pneumoniae (CIP-R SPN) using an in vitro model. J Antimicrob Chemoth, 2001, 47: 40
Yao S, Sheng SG, Cai JH, Zhang JS, Lin NY. Nanosecond pulse-radiolysis studies in China. Radiat Phys Chem, 1995, 46: 105–109
Lorenzo F, Navaratnam S, Edge R, Allen NS. Primary photophysical properties of moxifloxacin—A fluoroquinolone antibiotic. Photochem Photobiol, 2008, 84: 1118–1125
Lorenzo F, Navaratnam S, Edge R, Allen NS. Primary photoprocesses in a fluoroquinolone antibiotic sarafloxacin. Photochem Photobiol, 2009, 85: 886–894
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, H., Liu, Y., Tang, R. et al. Pulse radiolysis study on gatifloxacin — A fluoroquinolone antibiotic. Sci. China Chem. 55, 1358–1363 (2012). https://doi.org/10.1007/s11426-012-4643-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-012-4643-5