Abstract
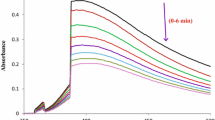
The kinetics of oxidation of moxifloxacin (MOX) was studied spectrophotometrically by a well-recognized analytical reagent, permanganate (Mn(VII), in aqueous alkaline medium at a constant ionic strength. The reaction was first order in [Mn(VII)] and less than unit order both in [MOX] and [alkali]. Retarding effect on rate of reaction was observed with an increase in ionic strength. The effect of dielectric constant of the medium was also studied. The multiple m/z values of ESI–MS spectra prove the existence of various oxidative products of MOX. The main product was identified as 1-cyclopropyl-6-fluoro-1,4-dihydro-7-(octahydro-2-oxopyrrolo[3,4-b] pyridin-6-yl)-8-methoxy-4-oxoquinoline-3-carboxylic acid. The other three oxidative products from MOX in the present study are similar to the oxidative products of other fluoroquinolones oxidations. However, the abnormally high values of m/z could be assigned to the permanganate complexes of the products, which are unusual in the non-metallic oxidation of MOX. A composite mechanism involving the monohydropermangante as the reactive species of the oxidant has been proposed. Activation parameters and thermodynamic parameters are calculated and the reaction constants involved in the different steps of the mechanisms are calculated.







Similar content being viewed by others
References
A. Carrington, M.C.R. Symons, Chem. Rev. 63, 443 (1963)
M. Jaky, L.I. Simandi, J. Chem. Soc. Perkin Trans. 2, 939 (1976)
P. Nath, K.K. Banerji, Indian J. Chem. 14, 660 (1976)
M. Jaky, J. Szammer, E. Simon-Trompler, J. Chem. Soc. Perkin Trans. 2, 1597 (2000)
P.K. Sen, P.R. Samaddar, K. Das, Transit. Met. Chem. 30, 907 (2005)
K. Mohnot, P.K. Sharma, K.K. Banerji, J. Org. Chem. 61, 1310 (1996)
S.A. Khan, P. Kumar, K. Saleem, Z. Khan, Colloids Surf. A: Physicochem. Eng. Asp. 302, 102 (2007)
E. Block, R. DeOrazio, M. Thiruvazhi, J. Org. Chem. 59, 2273 (1994)
N. Xie, R.A. Binstead, E. Block, W.D. Chandler, D.G. Lee, T.J. Meyer, M. Thiruvazhi, J. Org. Chem. 65, 1008 (2000)
A.R. Hajipour, S.E. Mallakpour, H. Adibi, Sulfur Lett. 25, 155 (2002)
P. Kumar, Z. Khan, Colloid Polym. Sci. 284, 1155 (2006)
N.N. Halligudi, S.M. Desai, S.T. Nandibewoor, Transit. Met. Chem. 26, 28 (2001)
R.G. Panari, R.B. Chougale, S.T. Nandibewoor, Polish J. Chem. 72, 99 (1998)
P.B. Addis, A.S. Csallany, S.E. Kindom, Some lipid oxidation products as xenobiotics, in Xenobiotics in Foods and Feeds, ed. by J.W. Finley, D.E. Schwass (American Chemical Society, Washington, DC, 1983), p. 85
J.R. Hanson, P.B. Hitchcock, S.J. Nagaratnam, J. Chem. Res. Synop. 22 (1999)
S. Dash, S. Patel, B.K. Mishra, Tetrahedron 65, 707 (2009)
L.I. Simandi, M. Jaky, Z.A. Schelley, J. Am. Chem. Soc. 107, 4220 (1985)
P.L. Timmanagoudar, G.A. Hiremath, S.T. Nandibewoor, Transit. Met. Chem. 22, 193 (1997)
U.S. Environmental Protection Agency. In Situ Remediation Technology: In Situ Chemical Oxidation EPA542-R-98-008; Office of Solid Waste and Emergency Response (U.S. EPA, Washington, DC, 1998)
R.L. Siegrist, M.A. Urynowicz, O.R. West, M.L. Crimi, K.S. Lowe, Principles and Practices of In Situ Chemical Oxidation Using Permanganate (Battelle, Columbus, OH, 2001)
Y. Eugeneyan, F.W. Schwartz, Environ. Sci. Technol. 34, 2535 (2000)
V.T. Andriole, The quinolones: Prospects, in The quinolones, 3rd edn., ed. by V.T. Andriole (Academic Press, London, 2000), p. 487
J.O. Maryadele, The Merck Index: An Encyclopedia of Chemicals, Drugs and Biologicals, 14th ed. (Merck Research Laboratories, Division of Merck and Co., Inc. Whitehouse Station, New Jersey, 2001), pp. 1125
British Pharmacopoeia, The Stationary Office Medicinal, Pharmaceutical Substances, London 2, 1401 (2009)
V.T. Andriole, Clin. Infect. Dis. 41, 113 (2005)
J.J. Champoux, Annu. Rev. Biochem. 70, 369 (2001)
USP DI., Vol. I, 21st edn. (Micromedex, New York, NY, 2001), pp. 1528
E. Miyazaki, M. Miyazaki, J.M. Chen, R.E. Chaisson, W.R. Bishai, Moxifloxacin (BAY12-8039). Antimicrob. Agents Chemother. 43, 85 (1999)
U. Hubicka, B. Zuromska-witek, J. Krzek, M. Walczak, M. Zylewski, Acta Poloniae Pharmaceut. Drug Res. 69, 821 (2012)
E.M. Golet, A.C. Alder, W. Giger, Environ. Sci. Technol. 36, 3645 (2002)
D.W. Kolpin, M. Skopec, M.T. Meyer, E.T. Furlong, S.D. Zaugg, Sci. Total Environ. 328, 119 (2004)
D.W. Kolpin, E.T. Furlong, M.T. Meyer, E.M. Thurman, S.D. Zaugg, L.B. Barber, H.T. Buxton, Environ. Sci. Technol. 36, 1202 (2002)
N.M. Vieno, H. Harkki, T. Tuhkanen, L. Kronberg, Environ. Sci. Technol. 41, 5077 (2007)
W. Xu, G. Zhang, X. Li, S. Zou, P. Li, Z. Hu, J. Li, Water Res. 41, 4526 (2007)
F. Tamtam, F. Mercier, B. Le Bot, J. Eurin, Q.T. Dinh, M. Clement, M. Chevreuil, Sci. Total Environ. 393, 84 (2008)
J.E. Renew, C.H. Huang, J. Chromatogr. A 1042, 113 (2004)
H. Nakata, K. Kannan, P.D. Jones, J.P. Giesy, Chemosphere 58, 759 (2005)
S. Castiglioni, R. Bagnati, R. Fanelli, F. Pomati, D. Calamari, E. Zuccato, Environ. Sci. Technol. 40, 357 (2006)
K.G. Karthikeyan, M.T. Meyer, Sci. Total Environ. 361, 196 (2006)
M. Seifrtova, A. Pena, C.M. Lino, P. Solich, Anal. Bioanal. Chem. 391, 799 (2008)
B. Wiedemann, H. Grimm, Susceptibility to antibiotics: species incidence and trends. in Antibiotics in Laboratory Medicine, ed. by V. Lorian (Williams and Wilkins, Baltimore, MD, 1996), pp. 900
P. Wang, H. Yi-Liang, Ching-Hua, Water Res. 44, 5989 (2010)
H. Zhang, C.H. Huang, The 3rd International Conference on Pharmaceuticals and Endocrine Disrupting Chemicals in Water (National Ground Water Association, Minneapolis, MN, 2003), pp. 19
H. Urszula, J. Krzek, B. Zuromska, M. Walczak, M. Zylewski, D. Pawlowski, Photochem. Photobiol. Sci. 11, 351 (2012)
R.E.L. Sheikh, A.S. Amin, A.A. Gouda, A.G. Youssef, Pharm. Anal. Acta. 240, 2153 (2013). doi:10.4172/2153-2435.1000240
W.F. El-Hawary, Kh Faisal, Al-Gethami. Eur. Chem. Bull. 2, 22 (2013)
A.I. Vogel, A text book of quantitative inorganic analysis, 4th edn. (ELBS, New York, 1978), p. 330
G.H. Jeffery, J. Bassett, J. Mendham, R.C. Denney, Vogel’s Text Book of Quantitative Chemical Analysis, 5th ed. (ELBS, Longman, Essex, 1996), pp. 371
R.G. Panari, R.B. Chougale, S.T. Nandibewoor, Oxidn. Commun. 21, 503 (1998)
D.C. Bilehal, R.M. Kulkarni, S.T. Nandibewoor, Can. J. Chem. 79, 1926 (2001)
G.H. Hugar, S.T. Nandibewoor, Transit. Met. Chem. 19, 215 (1994)
P. Naik, S.A. Chimatadar, S.T. Nandibewoor, Ind. Eng. Chem. Res. 48, 2548 (2009)
M. Jaky, I.V. Kozhevnikov, E. Hoft, Int. J. Chem. Kinet. 24, 1055 (1992)
K.A. Thabaj, S.D. Kulkarni, S.A. Chimatadar, S.T. Nandibewoor, Polyhedrone 26, 4877 (2007)
J. Sharma, D.K. Majumdar, Int. J. Pharm. Pharm. Sci. 4, 605 (2012)
Acknowledgments
The authors are grateful to the Principal, Karnatak Science College, Dharwad, Karnataka, India for providing the necessary facilities to carry out this work. They also thank the Raptakos Brett and Co., Microlabs Ltd. KLAB, Mumbai, India for providing the free sample of Moxifloxacin.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Badi, S.S., Tuwar, S.M. Permanganate oxidative products of moxifloxacin, a fluoroquinolone drug: a mechanistic approach. Res Chem Intermed 41, 7827–7845 (2015). https://doi.org/10.1007/s11164-014-1862-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-014-1862-8