Abstract
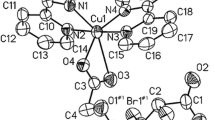
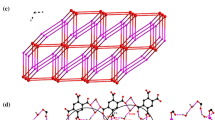
A discrete centrosymmetric (H2O)20(CH3OH)4 binary cluster was confined in the cavity of a metal-ligand hybrid [Ag4(bpda)2(bpp)4·14H2O·2CH3OH] n (1) (where bpp = 1,3-bis(4-pyridyl)propane and H2bpda = 2,2′-biphenyldicarboxylic acid). The novel mixed water-methanol cluster consists of one grail-shaped hexadecameric cluster, four dangling water and four hanging methanol molecules. The (H2O)16 cluster is composed of two pairs of edge-sharing (H2O)5 rings attached to one (H2O)4 core with twenty hydrogen bonds. Alternatively, the (H2O)16 cluster is structurally similar to a complicated hydrocarbon generated by undergoing [2+2] cycloaddition of 1,2,3,4,5,6-hexahydropentalene, which reveals the resemblance between water clusters and organic compounds.
Similar content being viewed by others
References
Eisenberg D, Kauzmann W. The Structure and Properties of Water. Oxford: Oxford University Press, 1969
Silverstein KAT, Haymet ADJ, Dill KA. A simple model of water and the hydrophobic effect. J Am Chem Soc, 1998, 120: 3166–3175
Janiak C, Scharmann TG, Mason SA. Two-dimensional water and ice layers: Neutron diffraction studies at 278, 263, and 20 K. J Am Chem Soc, 2002, 124: 14010–14011
Fletcher NH. The Chemical Physics of Ice. Cambridge: Cambridge University Press, 1970
Liu K, Brown MG, Carter C, Saykally RJ, Gregory JK, Clary DC. Characterization of a cage form of the water hexamer. Nature, 1996, 381: 501–503
Nauta K, Miller RE. Formation of cyclic water hexamer in liquid helium: the smallest piece of ice. Science, 2000, 287: 293–295
Kim J, Majumdar D, Lee HM, Kim KS. Structures and energetics of the water heptamer: Comparison with the water hexamer and octamer. J Chem Phys, 1999, 110: 9128–9134
Nangia A. ed. Encyclopaedia of Supramolecular Chemistry. New York: Taylor & Francis, 2007
Ludwig R. Water: From clusters to the bulk. Angew Chem Int Ed, 2001, 40: 1808–1827
Infantes L, Motherwell S. Water clusters in organic molecular crystals. CrystEngComm, 2002, 4: 454–461
Infantes L, Chisholm J, Motherwell S. Extended motifs from water and chemical functional groups in organic molecular crystals. CrystEngComm, 2003, 5: 480–486
Mascal M, Infantes L, Chisholm J. Water oligomers in crystal hydrates-what’s news and what isn’t? Angew Chem Int Ed, 2006, 45: 32–36
Xu WZ, Sun JL, Huang ZT, Zheng QY. Molecular encapsulation of a discrete (H2O)32 cluster with S6 symmetry in an organic crystalline supermolecule. Chem Commun, 2009, 171-173
Han ZB, Zhang GX, Zeng MH, Yuan DQ, Fang QR, Li JR, Ribas J, Zhou HC. Unprecedented marriage of a cationic pentanuclear cluster and a 2D polymeric anionic layer based on a flexible tripodal ligand and a CuII ion. Inorg Chem, 2010, 49: 769–771
Wei ML, He C, Hua WJ, Duan CY, Li SH, Meng QJ. A large protonated water cluster H+(H2O)27 in a 3D metal-organic framework. J Am Chem Soc, 1998, 120: 3166–3175
Wang Y, Okamura TA, Sun WY, Ueyama N. Large (H2O)56(OH)6 and (H2O)20 clusters inside a nanometer-sized M6L8 cage constructed by five-coordinated copper(II) and flexible carboxamide-containing tripodal ligand. Cryst Growth Des, 2008, 8: 802–804
Cao ML, Wu JJ, Mo HJ, Ye BH. Template trapping and crystal structure of the magic number (H2O)21 cluster in the tetrahedral hole of a nanoscale global ion packed in a face-centered cubic pattern. J Am Chem Soc, 2009, 131: 3458–3459
Teil SB, Gokavi GS, Sairam M, Aminabhavi TM. Mixed matrix membranes of poly(vinyl alcohol) loaded with phosphomolybdic heteropolyacid for the pervaporation separation of water-isopropanol mixtures. Colloids Surf, A Physicochem Eng Asp, 2007, 301: 55–62
Odriozola G, Schmitt A, Fernández JC, Álvarez RH. Aggregation kinetics of latex microspheres in alcohol-water media. J Colloid Interface Sci, 2007, 310: 471–480
Travers F, Douzou P. Dielectric constants of alcoholic-water mixtures at low temperature. J Phys Chem, 1970, 74: 2243–2244
Bender ML, Glasson WA. The kinetics of simultaneous hydrolysis and alcoholysis of esters in aqueous alcohol solution. J Am Chem Soc, 1959, 81: 1590–1597
Stockman PA, Blake GA, Lovas FJ, Suenran RD. Microwave rotation-tunneling spectroscopy of the water-methanol dimer: Direct structural proof for the strongest bound conformation. J Chem Phys, 1997, 107: 3782–3790
Mejía SM, Espinal JF, Mondragón F. Cooperative effects on the structure and stability of (ethanol)3-water, (methanol)3-water heterotetramers and (ethanol)4, (methanol)4 tetramers. J Mol Struct Theochem, 2009, 901: 186–193
da Silva JAB, Moreira FGB, dos Santos VML, Longo RL. On the hydrogen bond networks in the water-methanol mixtures: topology, percolation and small-world. Phys Chem Chem Phys, 2011, 13: 6452–6461
Laaksonen A, Kusalik PG, Svishchev IM. Three-dimensional structure in water-methanol mixture. J Phys Chem A, 1997, 10: 5910–5918
Wakisaka A, Abdoul-Carime H, Yamamoto Y, Kiyozumi Y. Non-ideality of binary mixtures water-methanol and water-acetonitrile from the viewpoint of clustering structure. J Chem Soc Faraday Trans, 1998, 94: 369–374
Raghuraman KN, Katti KK, Barbour LJ, Pillarsetty N, Barnes CL, Katti KV. Characterization of supramolecular (H2O)18 water morphology and water-methanol (H2O)15(CH3OH)3 clusters in a novel phosphorus functionalized trimeric amino acid host. J Am Chem Soc, 2003, 125: 6955–6961
Luo GG, Li DX, Wu DL, Liu L, Zhao QH, Peng C, Xiao ZJ, Dai JC. Characterization of a well-resolved acyclic methanol(water)5 heterohexamer in the solid state. Inorg Chem Commun, 2012, 17: 108–112
Luo GG, Xiong HB, Dai JC. Syntheses, structural characterization, and properties of {[Cu(bpp)2 (H2O)2](tp)·7H2O} and {[Cu(bpp)2(H2O)](ip)·7H2O} complexes. N New examples of the organic anionic template effect on induced assembly of water clusters (bpp = 1,3-Bis(4-pyridyl) propane, tp = terephthalate, ip = isophthalate). Cryst Growth Des, 2011, 11: 507–513
Luo GG, Xiong HB, Sun D, Wu DL, Huang RB, Dai JC. A discrete spirocyclic (H2O)9 cluster and 1D novel water chain with tetrameric and octameric clusters in cationic hosts. Cryst Growth Des, 2011, 11: 1948–1956
Luo GG, Wu DL, Liu L, Xia JX, Li DX, Dai JC, Xiao ZJ. A discrete water hexamer with a new planar tetrameric water moiety trapped in the crystal host of [Ag(azelate)(4,4′-bipyridine)]·(H2O)3. J Mol Struct, 2011, 1005: 172–177
He WJ, Luo GG, Wu DL, Liu L, Xia JX, Li DX, Dai JC, Xiao ZJ. An odd-numbered heptameric water cluster containing a puckered pentamer self-assembled in a Ag(I) polymeric solid. Inorg Chem Commun, 2012, 18: 4–7
Xiong HB, Sun D, Luo GG, Huang RB, Dai JC. One 1D T4(0)A(0) water tape embedded in a 1D Cu(II) coordination polymer with 1,3-bis(4-pyridyl)pro-pane. J Mol Struct, 2011, 990: 164–168
Luo GG, Wu DL, Liu L, Li DX, Zhao QH, Xiao ZJ, Dai JC. A hybrid carboxylate-water decamer with a discrete octameric water moiety self-assembled in a 2D copper(II) coordination polymer. 2012, doi: 10. 1007/s11426-012-4492-2.
Higashi T. ABSCOR. Empirical Absorption Correction based on Fourier Series Approximation. Tokyo: Rigaku Corporation, 1995
Sheldrick GM. SHELXS-97. Program for X-ray Crystal Structure Determination. Germany: University of Göttingen, 1997
Sheldrick GM. SHELXL-97. Program for X-ray Crystal Structure Refinement. Germany: University of Göttingen, 1997
Spek AL. Implemented as the PLATON Procedure, a Multipurpose Crystallographic Tool. The Netherlands: Utrecht University, 1998
Brandenburg K. DIAMOND, Version 3.1f. Crystal Impact GbR, Bonn, Germany
Yang L, Powell DR, Houser RP. Structural variation in copper(I) complexes with pyridylmethylamide ligands: Structural analysis with a new four-coordinate geometry index, τ4. Dalton Trans, 2007, 955-964
Brandys MC, Puddephatt RJ. Ring, polymer and network structures in silver(I) complexes with dipyridyl and diphosphine ligands. Chem Commun, 2001, 1508–1509
Cordes DB, Hanton LR. Structural invariance in silver(I) coordination networks formed using flexible four-armed thiopyridine ligands. Inorg Chem, 2007, 46: 1634–1644
Ghosh SK. Structure of a discrete hexadecameric water cluster in a metal-organic framework structure. Inorg Chem, 2004, 43: 6687–6889
Yoo S, Aprà E, Zeng XC, Xantheas SS. High-level ab initio electronic structure calculations of water clusters (H2O)16 and (H2O)17: A new global minimum for (H2O)16. J Phys Chem Lett, 2010, 1: 3122–3127
Bi YF, Liao WP, Zhang HJ, Li DQ. Assembly of “discrete” (H2O)16 water clusters within a supramolecular compound of calixarene. CrystEngComm, 2009, 11: 1213–1216
Neogi S, Sañudo EC, Bharadwaj PK. Transition-metal porous coordination polymers with a podand ligand: structure of discrete water clusters and variable-temperature magnetism. Eur J Inorg Chem, 2007, 5426-5432
Li JY, Yu JH, Xu RR. A unique self-assembled (H2O)16 water cluster in an inorganic crystal host. Phys Chem Chem Phys, 2009, 11: 1291–1293
Barbour LJ, Orr GW, Atwood JL. An intermolecular (H2O)10 cluster in a solid-state supramolecular complex. Nature, 1998, 393: 671–673
Barbour LJ, Orr GW, Atwood JL. Characterization of a well resolved supramolecular ice-like (H2O)10 cluster in the solid state. Chem Commun, 2000, 859-860
Matsumoto M, Saito S, Ohmine I. Molecular dynamics simulation of the ice nucleation and growth process leading to water freezing. Nature, 2002, 416: 409–413
Custalcean R, Afloroaiei C, Vlassa M, Polverejan M. Formation of extended tapes of cyclic water hexamer in an organic molecular crystal host. Angew Chem Int Ed, 2000, 39: 3094–3096
Maheshwary S, Patel N, Kulkarni AD, Gadre SR. Structure and stability of water clusters (H2O)n, n = 8-20: An ab initio investigation. J Phys Chem A, 2001, 105: 10525–10537
Sang RL, Xu L. Reversible formation of regular pentagonal dodecahedral (H2O)20 in a 2D metal-organic framework. CrystEngComm, 2010, 12: 1377–1381
Jeffrey GA. An Introduction to Hydrogen Bonding. New York: Oxford University Press, 1997, Chapters 8 and 9.
González L, Mó O, Yáñes M. High level ab initio and density functional theory studies on methanol-water dimmers and cyclic methanol(water)2 trimer. J Chem Phys, 1998, 109: 139–150
Kirschner KN, Woods RJ. Quantum mechanical study of the nonbonded forces in water-methanol complexes. J Phys Chem A, 2001, 105: 4150–4155
Clary DC, Benoit DM, Mourik ATV. H-densities: A new concept for hydrated molecules. Acc Chem Res, 2000, 33: 441–447
Fileti EE, Chaudhuri P, Canuto S. Relative strength of hydrogen bond interaction in alcohol-water complexes. Chem Phys Lett, 2004, 400: 494–499
Mondal R, Howard JAK. A structural investigation of six solvates of trans-1,4-bis(phenylethynyl)-cyclohexane-1,4-diol. Cryst Growth Des, 2008, 8: 4359–4366
de Almeida ET, Mauro AE, Santana AM, Ananias SR, Netto AVG, Ferreira JG, Santos RHA. Self-assembly of organometallic Pd(II) complexes via CH3…π interactions: The first example of a cyclopalladated compound with herringbone stacking pattern. Inorg Chem Commun, 2007, 10: 1394–1398
Nakamoto K. Infrared and Raman Spectra of Inorganic and Coordination Compounds. New York: John Wiley & Sons, 1986
Wu H, Dong XW, Liu HY, Ma JF, Li SL, Yang J, Liu YY, Su ZM. Influence of anionic sulfonate-containing co-ligands on the solid structures of silver complexes supported by 4,4′-bipyridine bridge. Dalton Trans, 2008, 5331–5341
Yin PX, Zhang J, Li ZJ, Qin YY, Cheng JK, Zhang L, Lin QP, Yao YG. Supramolecular isomerism and various chain/layer substructures in silver(I) compounds: syntheses, structures, and luminescent properties. Cryst Growth Des, 2009, 9: 4884–4896
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Luo, G., He, W., Liu, L. et al. A self-assembled (H2O)20(CH3OH)4 binary cluster containing a grail-shaped hexadecameric water cluster trapped in the cavity of a metal-ligand hybrid. Sci. China Chem. 55, 2507–2514 (2012). https://doi.org/10.1007/s11426-012-4566-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-012-4566-1