Abstract
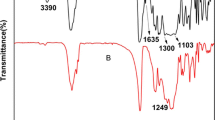
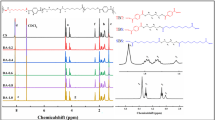
The aminolysis can effectively introduce primary amine (−NH2) groups onto polyester materials, enabling a variety of subsequent surface biofunctionalization reactions. However, less attention has been paid to the basic knowledge of aminolysis reaction in terms of reaction kinetics and its influences on materials properties. In this study, taking the widely used poly(ɛ-caprolactone) (PCL) as a typical example, the influences of diamines and solvent property on the surface −NH2 density are firstly assessed by using X-ray photoelectron spectroscopy (XPS) and colorimetric analysis. Results show that smaller diamine molecules and nonpolar alcohols could accelerate the reaction. The reaction kinetics with 1,6-hexanediamine is further investigated as a function of temperature, reaction time, and diamine concentration. During the initial stage, the reaction shows a 1st order kinetics with the diamine concentration and has an activation energy of 54.5 kJ/mol. Ionization state of the −NH2 groups on the PCL surface is determined, revealing that the pKa of −NH3 + (<5) is much lower than that of the corresponding diamine molecules in solution. After aminolysis, surface hydrophilicity of PCL membrane is significantly enhanced, while surface elastic modulus and average molecular weight are decreased to some extent, and others such as weight, surface morphology and bulk mechanical strength are not apparently changed. The introduced −NH2 groups are found to be largely lost at 37 °C, but can be mostly maintained at low temperature.
Similar content being viewed by others
References
Morent R, De Geyter N, Desmet T, Dubruel P, Leys C. Plasma surface modification of biodegradable polymers: A review. Plasm Proc Polym, 2011, 8(3): 171–190
Jiao YP, Cui FZ. Surface modification of polyester biomaterials for tissue engineering. Biomed Mater, 2007, 2(4): R24–R37
Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science, 1997, 276(5317): 1425–1428
Kilian KA, Bugarija B, Lahn BT, Mrksich M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc Natl Acad Sci USA, 2010, 107(11): 4872–4877
Dalby MJ, Gadegaard N, Tare R, Andar A, Riehle MO, Herzyk P, Wilkinson CD, Oreffo RC. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat Mater, 2007, 6(12): 997–1003
Du J, Chen X, Liang X, Zhang G, Xu J, He L, Zhan Q, Feng XQ, Chien S, Yang, C. Integrin activation and internalization on soft ECM as a mechanism of induction of stem cell differentiation by ECM elasticity. Proc Natl Acad Sci USA, 2011, 108(23): 9466–9471
Cameron AR, Frith JE, Cooper-White JJ. The influence of substrate creep on mesenchymal stem cell behaviour and phenotype. Biomaterials. 2011, 32(26): 5979–5993
Ayala R, Zhang C, Yang D, Hwang Y, Aung A, Shroff SS, Arce FT, Lal R, Arya G, Varghese S. Engineering the cell-material interface for controlling stem cell adhesion, migration, and differentiation. Biomaterials, 2011, 32(15): 3700–3711
Quirk RA, Chan WC, Davies MC, Tendler SJ, Shakesheff KM. Poly(L-lysine)-GRGDS as a biomimetic surface modifier for poly(lactic acid). Biomaterials, 2001, 22(8): 865–872
Yang J, Bei J, Wang S. Enhanced cell affinity of poly (D,L-lactide) by combining plasma treatment with collagen anchorage. Biomaterials, 2002, 23(12): 2607–2614
Cai K, Yao K, Lin S, Yang Z, Li X, Xie H, Qing T, Gao L. Poly(D,L-lactic acid) surfaces modified by silk fibroin: Effects on the culture of osteoblast in vitro. Biomaterials, 2002, 23(4): 1153–1160
Goddard JM, Hotchkiss JH. Polymer surface modification for the attachment of bioactive compounds. Prog Polym Sci, 2007, 32(7): 698–725
Kim MS, Khang G, Lee HB. Gradient polymer surfaces for biomedical applications. Prog Polym Sci, 2008, 33(1): 138–164
Hersel U, Dahmen C, Kessler H. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials, 2003, 24(24): 4385–4415
Cipitria A, Skelton A, Dargaville TR, Dalton PD, Hutmacher DW. Design, fabrication and characterization of PCL electrospun scaffolds — A review. J Mater Chem, 2011, 21(26): 9419–9453
Zhu YB, Gao CY, Shen JC. Surface modification of polycaprolactone with poly(methacrylic acid) and gelatin covalent immobilization for promoting its cytocompatibility. Biomaterials, 2002, 23(24): 4889–4895
Zhu YB, Gao CY, Liu XY, Shen JC. Surface modification of polycaprolactone membrane via aminolysis and biomacromolecule immobilization for promoting cytocompatibility of human endothelial cells. Biomacromolecules, 2002, 3(6): 1312–1319
Zhu YB, Gao CY, He T, Shen JC. Endothelium regeneration on luminal surface of polyurethane vascular scaffold modified with diamine and covalently grafted with gelatin. Biomaterials, 2004, 25(3): 423–430
Zhu YB, Gao CY, Liu XY, He T, Shen JC. Immobilization of biomacromolecules onto aminolyzed poly(L-lactic acid) toward acceleration of endothelium regeneration. Tissue Eng, 2004, 10(1–2): 53–61
Balmayor ER, Pashkuleva I, Frias AM, Azevedo HS, Reis RL. Synthesis and functionalization of superparamagnetic poly-epsilon-caprolactone microparticles for the selective isolation of subpopulations of human adipose-derived stem cells. J R Soc Interface, 2011, 8(59): 896–908
Zhang HN, Lin CY, Hollister SJ. The interaction between bone marrow stromal cells and RGD-modified three-dimensional porous polycaprolactone scaffolds. Biomaterials, 2009, 30(25): 4063–4069
Causa F, Battista E, Della Moglie R, Guarnieri D, Iannone M, Netti PA. Surface investigation on biomimetic materials to control cell adhesion: The case of RGD conjugation on PCL. Langmuir, 2010, 26(12): 9875–9884
Gumusderelioglu M, Karakecili A, Demirtas TT. Osteogenic activities of MC3T3-E1 cells on heparin-immobilized poly(caprolactone) membranes. J Bioact Compat Polym, 2011, 26(3): 257–269
Jiang H, Wang XB, Li CY, Li JS, Xu FJ, Mao C, Yang WT, Shen J. Improvement of hemocompatibility of polycaprolactone film surfaces with zwitterionic polymer brushes. Langmuir, 2011, 27(18): 11575–11581
Hong Y, Gao CY, Xie Y, Gong YH, Shen JC. Collagen-coated polylactide microspheres as chondrocyte microcarriers. Biomaterials, 2005, 26(32): 6305–6313
Mattanavee W, Suwantong O, Puthong S, Bunaprasert T, Hoven VP, Supaphol P. Immobilization of biomolecules on the surface of electrospun polycaprolactone fibrous scaffolds for tissue engineering. ACS Appl Mater Interface, 2009, 1(5): 1076–1085
Zhang H, Lin CY, Hollister SJ. The interaction between bone marrow stromal cells and RGD-modified three-dimensional porous polycaprolactone scaffolds. Biomaterials, 2009, 30(25): 4063–4069
Li CY, Yuan W, Jiang H, Li JS, Xu FJ, Yang WT, Ma J. PCL film surfaces conjugated with P(DMAEMA)/gelatin complexes for improving cell immobilization and gene transfection. Bioconjug Chem, 2011, 22(9): 1842–1851
Xu FJ, Yang XC, Li CY, Yang WT. Functionalized polylactide film surfaces via surface-initiated ATRP. Macromolecules, 2011, 44(7): 2371–2377
Glasoe PK, Kleinberg J, Audrieth LF. Acid catalysis in amines. II. The catalytic effect of various butylammonium salts on the aminolysis of ethyl phenylacetate in anhydrous n-butylamine. J Am Chem Soc, 1939, 61(9): 2387–2389
Hawkins PJ, Piscalnikow I. A kinetic study of the aminolysis and hydrolysis of alpha-naphthyl acetate. J Am Chem Soc, 1955, 77(10): 2771–2773
Bunnett JF, Davis GT. The mechanism of aminolysis of esters1,2. J Am Chem Soc, 1960, 82(3): 665–674
Betts RL, Hammett LP. A kinetic study of the ammonolysis of phenylacetic esters in methanol solution. J Am Chem Soc, 1937, 59(8): 1568–1572
Bender ML. Oxygen exchange as evidence for the existence of an intermediate in ester hydrolysis. J Am Chem Soc, 1951, 73(4): 1626–1629
Watanabe WH, Defonso LR. The kinetics and mechanism of the aminolysis of ethyl formate with normal-butylamine. J Am Chem Soc, 1956, 78(18): 4542–4549
Avadanei M, Drobota M, Stoica I, Rusu E, Barboiu V. Surface morphology and amide concentration depth profile of aminolyzed poly(ethylene terephthalate) films. J Polym Sci Polym Chem, 2010, 48(23): 5456–5467
Bech L, Meylheuc T, Lepoittevin B, Roger P. Chemical surface modification of poly(ethylene terephthalate) fibers by aminolysis and grafting of carbohydrates. J Polym Sci Polym Chem, 2007, 45(11): 2172–2183
Griesser HJ, Chatelier RC, Gengenbach TR, Johnson G, Steele JG. Growth of human cells on plasma polymers: putative role of amine and amide groups. J Biomater Sci Polym Ed, 1994, 5(6): 531–554
Tang ZG, Black RA, Curran JM, Hunt JA, Rhodes NP, Williams DF. Surface properties and biocompatibility of solvent-cast poly(ɛ-caprolactone) films. Biomaterials, 2004, 25(19): 4741–4748
Ma ZW, Mao ZW, Gao CY. Surface modification and property analysis of biomedical polymers used for tissue engineering. Colloids Surf B: Biointerfaces, 2007, 60(2): 137–157
Vezenov DV, Noy A, Rozsnyai LF, Lieber CM. Force titrations and ionization state sensitive imaging of functional groups in aqueous solutions by chemical force microscopy. J Am Chem Soc, 1997, 119(8): 2006–2015
Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell, 2006, 126(4): 677–689
Piskin E. Biodegradable polymers as biomaterials. J Biomater Sci Polym Ed, 1995, 6(9): 775–795
Woodruff MA, Hutmacher DW. The return of a forgotten polymer — Polycaprolactone in the 21st century. Prog Polym Sci, 2010, 35(10): 1217–1256
Chatelier RC, Xie XM, Gengenbach TR, Griesser HJ. Effects of plasma modification conditions on surface restructuring. Langmuir, 1995, 11(7): 2585–2591
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, Y., Mao, Z., Shi, H. et al. In-depth study on aminolysis of poly(ɛ-caprolactone): Back to the fundamentals. Sci. China Chem. 55, 2419–2427 (2012). https://doi.org/10.1007/s11426-012-4540-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-012-4540-y