Abstract
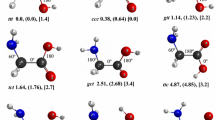
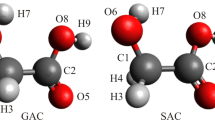
We present a systematic study of 1:1 glycine-water complexes involving all possible glycine conformers. The complex geometries are fully optimized for the first time both in the gas phase and in solution using three DFT methods (B3LYP, PBE1PBE, X3LYP) and the MP2 method. We calculate the G3 energies and use them as the reference data to gauge hydrogen bond strength in the gas phase. The solvent effects are treated via the integral equation formalism-polarizable continuum model (IEF-PCM). Altogether, we locate fifty-two unique nonionized (N) structures and six zwitterionic (Z) structures in the gas phase, and fifty-five N structures and thirteen Z structures in solution. Both correlation and solvation are shown to be important in geometry determination. We found that in the gas phase, a water molecule binds more strongly to the carboxylic acid group of glycine than to its amine group, whereas in solution phase the reverse is true. The most stable Z structure is isoenergetic with the most stable N structure.
Similar content being viewed by others
References
Zhang H, Zhou Z, Shi Y. Density functional theory study of the hydrogen-bonding interaction of 1:1 complexes of alanine with water. J Phys Chem A, 2004, 108: 6735–6743
Degtyarenko IM, Jalkanen KJ, Gurtovenko AA, Nieminen RM. L-alanine in a droplet of water: A density-functional molecular dynamics study. J Phys Chem B, 2007, 111: 4227–4234
Ji N, Shen YR. Sum frequency vibrational spectroscopy of leucine molecules adsorbed at air-water interface. J Chem Phys, 2004, 120: 7107–7112
Zhang P, Han SH, Zhang Y, Ford RC, Li JC. Neutron spectroscopic and Raman studies of interaction between water and proline. Chem Phys, 2008, 345: 196–199
Furić K, Mohaček V, Bonifačić M, Štefanić I. Raman-spectroscopic study of H2O and D2O water solutions of glycine. J Mol Struct, 1992, 267: 39–44
Khurgin YI, Kudryashova VA, Zavizion VA. Study of intermolecular interactions in aqueous solutions by millimeter spectroscopy. 6. Negative hydration of the glycine zwitterions. Russ Chem Bull, 1997, 46: 1248–1250
Sasaki M, Kameda Y, Yaegashi M, Usuki T. Hydration structure around the methylene group of glycine molecule. Bull Chem Soc Jpn, 2003, 76: 2293–2299
Kameda Y, Ebata H, Usuki T, Uemura O, Misawa M. Hydration structure of glycine molecules in concentrated aqueous solutions. Bull Chem Soc Jpn, 1994, 67: 3159–3164
Parsons MT, Koga Y. Hydration number of glycine in aqueous solution: An experimental estimate. J Chem Phys, 2005, 123: 234504
Alonso JL, Cocinero EJ, Lesarri A, Sanz ME, Lopez JC. The glycine-water complex. Angew Chem Int Ed, 2006, 45: 3471–3474
Ramaekers R, Pajak J, Lambie B, Maes G. Neutral and zwitterionic glycine.H2O complexes: A theoretical and matrix-isolation Fourier transform infrared study. J Chem Phys, 2004, 120: 4182–4193
Jensen JH, Gordon MS. On the number of water molecules necessary to stabilize the glycine zwitterion. J Am Chem Soc, 1995, 117: 8159–8170
Basch H, Stevens WJ. The structure of glycine water H-bonded complexes. Chem Phys Lett, 1990, 169: 275–280
Tortonda FR, Pascual-Ahuir JL, Silla E, Tuñón I. Why is glycine a zwitterion in aqueous solution? A theoretical study of solvent stabilizing factors. Chem Phys Lett, 1996, 260: 21–26
Wang WZ, Zheng WX, Pu XM, Wong NB, Tian AM. The 1:1 glycine-water complex: some theoretical observations. J Mol Struct (Theochem), 2002, 618: 235–244
Wang WZ, Pu XM, Zheng WX, Wong NB, Tian AM. Some theoretical observations on the 1:1 glycine zwitterion-water complex. J Mol Struct (Theochem), 2003, 626: 127–132
Twari S, Mishra PC, Suhai S. Solvent effect of aqueous media on properties of glycine: Significance of specific and bulk solvent effects, and geometry optimization in aqueous media. Int J Quantum Chem, 2008, 108: 1004–1016
Bachrach SM. Microsolvation of glycine: A DFT study. J Phys Chem A, 2008, 112: 3722–3730
Aikens CM, Gordon MS. Incremental solvation of nonionized and zwitterionic glycine. J Am Chem Soc, 2006, 128: 12835–12850
Mezei M, Mehrotra PK, Beveridge DL. Monte Carlo computer simulation of the aqueous hydration of the glycine zwitterion at 25 °C. J Biomol Struct Dyn, 1984, 2: 1–27
Ding Y, Krogh-Jespersen K. The 1:1 glycine zwitterion-water complex: An ab initio electronic structure study. J Comput Chem, 1996, 17: 338–349
Bandyopadhyay P. Gordon MS. A combined discrete/continuum solvation model: Application to glycine. J Chem Phys, 2000, 113: 1104–1109
Bandyopadhyay P, Gordon MS, Mennucci B, Tomasi J. An integrated effective fragment-polarizable continuum approach to solvation: Theory and application to glycine. J Chem Phys, 2002, 116: 5023–5032
Cui Q. Combining implicit solvation models with hybrid quantum mechanical/molecular mechanical methods: A critical test with glycine. J Chem Phys, 2002, 117: 4720–4728
Rzepa HS, Yi MY. An AM1 and PM3 molecular orbital and self-consistent reaction-field study of the aqueous solvation of glycine, alanine and proline in their neutral and zwitterionic forms. J Chem Soc Perkin Trans 2, 1991, 531–537
Iijima K, Tanaka K, Onuma S. Main conformer of gaseous glycine: molecular structure and rotational barrier from electron diffraction data and rotational constants. J Mol Struct, 1991, 246: 257–266
Suenram RD, Lovas FJ. Millimeter wave spectrum of glycine. A new conformer. J Am Chem Soc, 1980, 102: 7180–7184
Albrecht G, Corey RB. The crystal structure of glycine. J Am Chem Soc, 1939, 61: 1087–1103
Marsh RE. A refinement of the crystal structure of glycine. Acta Crystallogr, 1958, 11: 654–663
Jönsson PG, Kvick Å. Precision neutron diffraction structure determination of protein and nucleic acid components. III. The crystal and molecular structure of the amino acid α-glycine. Acta Crystallogr Sect B, 1972, 28: 1827–1833
Hückel W. Theoretical Principles of Organic Chemistry. Vol II. New York: Elsevier, 1958
Diken EG, Hammer NI, Johnson MA. Preparation and photoelectron spectrum of the glycine molecular anion: Assignment to a dipole-bound electron species with a high-dipole moment, non-zwitterionic form of the neutral core. J Chem Phys, 2004, 120, 9899–9902
Teh CK, Sipior J, Sulkes M. Spectroscopy of tryptophan in supersonic expansions—addition of solvent molecules. J Phys Chem, 1989, 93, 5393–5400
Lee C, Yang W, Parr RG. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B, 1988, 37: 785–789
Becke AD. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A, 1988, 38: 3098–3100
Becke AD. Density-functional for thermochemistry. III. The role of exact exchange. J Chem Phys, 1993, 98: 5648–5652
Burke K, Ernzerhof M, Perdew JP. Generalized gradient approximation made simple. Phys Rev Lett, 1996, 77: 3865–3868
Adamo C, Barone V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J Chem Phys, 1999, 110: 6158–6170
Xu X, Zhang QS, Muller RP, Goddard III W A. An extended hybrid density functional X3LYP with improved descriptions of nonbond interactions and thermodynamic properties of molecular systems. J Chem Phys, 2005, 122: 014105
Xu X, Goddard III WA. The X3LYP extended density functional for accurate descriptions of nonbond interactions, spin states, and thermochemical properties. Proc Natl Acad Sci USA, 2004, 101: 2673–2677
Xu X, Goddard III WA. Bonding properties of the water dimer: A comparative study of density functional theories. J Phys Chem A, 2004, 108: 2305–2313
Su JT, Xu X, Goddard III WA. Accurate energies and structures for large water clusters using the X3LYP hybrid density functional. J Phys Chem A, 2004, 108: 10518–10526
Curtiss LA, Raghavachari K, Redfern PC, Rassolov V, Pople JA. Gaussian-3 (G3) theory for molecules containing first and second-row atoms. J Chem Phys, 1998, 109: 7764–7776
Mennucci B, Tomasi J. Continuum solvation models: A new approach to the problem of solute’s charge distribution and cavity boundaries. J Chem Phys, 1997, 106: 5151–5158
Cancès MT, Mennucci B, Tomasi J. A new integral equation formalism for the polarizable continuum model: Theoretical background and applications to isotropic and anisotropic dielectrics. J Chem Phys, 1997, 107: 3032–3041
Cossi M, Barone V, Mennucci B, Tomasi J. Ab initio study of ionic solutions by a polarizable continuum dielectric model. Chem Phys Lett, 1998, 286: 253–260
Császár AG. Conformers of gaseous glycine. J Am Chem Soc, 1992, 114: 9568–9575
Hariharan PC, Pople JA. The influence of polarization functions on molecular orbital hydrogenation energies. Theor Chim Acta, 1973, 28: 213–222
Clark T, Chandrasekhar J, Spitznagel GW, Schleyer P v R. Efficient diffuse function-augmented basis sets for anion calculations. III. The 3–21+G basis set for first-row elements, Li-F. J Comput Chem, 1983, 4: 294–301
Woon DE, Dunning TH. Gaussian basis sets for use in correlated molecular calculations. III. The atomic aluminum through argon. J Chem Phys, 1993, 98: 1358–1371
Kendall RA, Dunning TH, Harrison RJ. Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J Chem Phys, 1992, 96: 6796–6806
Ke HW, Rao L, Xu X, Yan YJ. Theoretical study of glycine conformers. J Theor Comp Chem, 2008, 7: 889–909
Rao L, Ke HW, Gang F, Xu X, Yan YJ. Performance of several density functional theory methods on describing hydrogen-bond interactions. J Comp Theor Chem, 2009, 5: 86–96
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JAJr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA. Gaussian 03, revision D.01, Gaussian, Inc., Wallingford CT, 2004
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Ke, H., Rao, L., Xu, X. et al. Density functional theory study of 1:1 glycine-water complexes in the gas phase and in solution. Sci. China Chem. 53, 383–395 (2010). https://doi.org/10.1007/s11426-010-0065-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-010-0065-4