Abstract
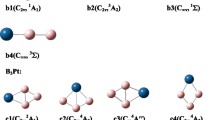
The interconversions between isomers with the same spin multiplicity of neutral B6 and charged B6 − and B6 + clusters have been investigated at the B3LYP/6-311+G* level of theory, including determination of the minimum energy pathways with transition states connecting the corresponding reactants and products. In dynamic calculations, 26 isomers were optimized, including 11 novel isomers. In order to further refine the energies, single-point B3LYP/6-311+G(3df) calculations were carried out on the corresponding B3LYP/6-311+G* geometries of all isomers of B6, B6 − and B6 + and the corresponding isomerization transition states. The stability of each isomer of B6 (singlet and triplet states), B6 − (doublet state) and B6 + (doublet state) was analyzed from both thermodynamic and dynamic viewpoints.
Similar content being viewed by others
References
Adams RM. Boron, Metallo-Boron Compounds, and Boranes. New York: Interscience Publishers, 1964
Matkovich VI. Boron and Refractory Borides. Berlin: Springer-Verlag, 1977
He J, Wu E, Wang H, Liu R, Tian Y. Ionicities of boron-boron bonds in B12 icosahedra. Phys Rev Lett, 2005, 94: 015504
Szwacki NG, Sadrzadeh A, Yakobson BI. B80 fullerene: An ab initio prediction of geometry, stability, and electronic structure. Phys Rev Lett, 2007 98: 166804
Szwacki NG, Sadrzadeh A, Yakobson BI. Erratum: B80 fullerene: An ab initio prediction of geometry, stability, and electronic structure. Phys Rev Lett, 2008, 100: 159901
Oger E, Crawford NRM, Kelting R, Weis P, Kappes MM, Ahlrichs R. Boron cluster cations: Transition from planar to cylindrical structures. Angew Chem Int Ed, 2007, 46: 8503–8506
Demirbas A. Hydrogen and boron as recent alternative motor fuels. Energy Sources, 2005, 27: 741–748
Eremets MI, Struzhikin VV, Mao H, Hemley RJ. Superconductivity in boron. Science, 2001, 293: 272–274
Reisch MS. High-performance fibers find expanding military, industrial uses. Chem Eng News, 1987, 65: 9–14
Hanley L, Whitten JL, Anderson SL. Collision-induced dissociation and ab initio studies of boron cluster ions: determination of structures and stabilities. J Phys Chem, 1988, 92: 5803–5812
Ray AK, Howard IA, Kanal KM. Structure and binding in small neutral and cationic boron clusters. Phys Rev B, 1992, 45: 14247–14255
Kato H, Tanaka E. Stabilities of small Ben and Bn clusters (4≤n≤8) by vibrational analysis. J Comput Chem, 1991, 12: 1097–1109
Boustani I. Systematic ab initio investigation of bare boron clusters: Determination of the geometry and electronic structures of Bn (n=2−14). Phys Rev B, 1997, 55: 16426–16438
Zhai HJ, Wang LS, Alexandrova AN, Boldyrev AI, Zakrzewski VG. Photoelectron spectroscopy and ab initio study of B3 − and B4 − anions and their neutrals. J Phys Chem A, 2003, 107: 9319–9328
Jin HW, Li QS. Structure and stability of B4, B4 + and B4 − clusters. Phys Chem Chem Phys, 2003, 5: 1110–1115
Zhai HJ, Wang LS, Alexandrova AN, Boldyrev AI. Electronic structure and chemical bonding of B5 − and B5 by photoelectron spectroscopy and ab initio calculations. J Chem Phys, 2002, 117: 7917–7924
Li QS, Jin HW. Structure and stability of B5, B5 +, and B5 − clusters. J Phys Chem A, 2002, 106: 7042–7047
Alexandrova AN, Boldyrev AI, Zhai HJ, Wang LS, Steiner E, Fowler P W. Structure and bonding in B6 − and B6: planarity and antiaromaticity. J Phys Chem A, 2003, 107: 1359–1369
Li QS, Jin Q, Luo Q, Tang AQ, Yu JK, Zhang HX. Structure and stability of B6, B6 + and B6 − clusters. Int J Quantum Chem, 2003, 94: 269–278
Ma J, Li Z, Fan K, Zhou M. Density functional theory study of the B6, B6 +, B6 −, and B6 2− clusters. Chem Phys Lett, 2003, 372: 708–716
Li QS, Gong LF, Gao ZM. Structures and stabilities of B7, B7 + and B7 − clusters. Chem Phys Lett, 2004, 390: 220–227
Alexandrova AN, Boldyrev AI, Zhai HJ, Wang LS. Electronic structure, isomerism, and chemical bonding in B7 − and B7. J Phys Chem A, 2004, 108: 3509–3517
Li QS, Zhao Y, Xu WG, Li N. Structure and stability of B8 − clusters. Int J Quantum Chem, 2005, 101: 219–229
Alexandrova AN, Boldyrev AI, Zhai HJ, Wang LS. All-boron aromatic clusters as potential new inorganic ligands and building blocks in chemistry. Coord Chem Rev, 2006, 250: 2811–2866
Niu J, Rao BK, Jena PJ. Atomic and electronic structures of neutral and charged boron and boron-rich clusters. J Chem Phys, 1997, 107: 132–140
Park JH, Lee JI, Kim MK, Oh YK, Cho HS. Ab-initio study of the structure and the binding in small boron clusters. J Korean Phys Soc, 1999, 34: 268–274
Parr RG, Yang W. Density-functional Theory of Atoms and Molecules. Oxford: Oxford University Press, 1989
Becke AD. Density-functional thermochemistry III. The role of exact exchange. J Chem Phys, 1993, 98: 5648–5652
Gonzalez C, Schlegel HB. An improved algorithm for reaction path following. J Chem Phys, 1989, 90: 2154–2161
Gonzalez C, Schlegel HB. Reaction path following in mass-weighted internal coordinates. J Phys Chem, 1990, 94: 5523–5527
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA. Gaussian03; Pittsburgh PA: Gaussian, Inc., 2003
Kato H, Yamashita K, Morokuma K. Ab initio MO study of neutral and cationic boron clusters. Chem Phys Lett, 1992, 190: 361–366
Ricca A, Bauschlicher CW. The structure and stability of Bn + clusters. Chem Phys, 1996, 208: 233–242
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wei, G., Pu, Z., Zou, R. et al. Isomerization of B6, B6 − and B6 + clusters. Sci. China Chem. 53, 202–209 (2010). https://doi.org/10.1007/s11426-010-0036-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-010-0036-9