Abstract
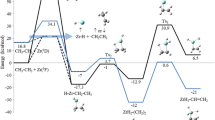
The mechanism of the spin-forbidden reaction Ti+(4F, 3d24s1) + C2H4→TiC2H2 + (2A2) + H2 on both doublet and quartet potential energy surfaces has been investigated at the B3LYP level of theory. Crossing points between the potential energy surfaces and the possible spin inversion process are discussed by means of spin-orbit coupling (SOC) calculations. The strength of the SOC between the low-lying quartet state and the doublet state is 59.3 cm−1 in the intermediate complex IM1-4B2. Thus, the changes of its spin multiplicity may occur from the quartet to the doublet surface to form IM1-2A1, leading to a sig-nificant decrease in the barrier height on the quartet PES. After the insertion intermediate IM2, two distinct reaction paths on the doublet PES have been found, i.e., a stepwise path and a concerted path. The latter is found to be the lowest energy path on the doublet PES to exothermic TiC2H2 +(2A2) + H2 products, with the active barrier of 4.52 kcal/mol. In other words, this reaction proceeds in the following way: Ti++C2H4→4IC→IM1-4B2→4,2ISC→IM1-2A1→[2TSins]→IM2→[2TSMCTS]→IM5→TiC2H2 +(2A2)+H2.
Similar content being viewed by others
References
Freiser B S. Ed. Organometallic Ion Chemistry. The Netherlands: Kiuwer Academic Publisher, 1996
Eller K, Schwarz H. Organometallic chemistry in the gas phase. Chem Rev, 1991, 91(6): 1121–1177
Weisshaar J C. Bare transition metal atoms in the gas phase: Reactions of M, M+, and M2+ with hydrocarbons. Acc Chem Res, 1993, 26(4): 213–219
Aristov N, Armentrout P B. Reaction mechanisms and thermochemistry of vanadium ions with ethane, ethene and ethyne. J Am Chem Soc, 1986, 108(8): 1806–1819
van Koppen P A M, Kemper P R, Bowers M T. Electronic state-selected reactivity of transition metal ions: cobalt(+) and iron(+) with propane. J Am Chem Soc, 1992, 114(27): 10941–10950
van Koppen P A M, Kemper P R, Bowers M T, Fisher E R, Armentrout P B. Relative energetics of C-H and C-C bond activation of alkanes: Reactions of Ni+ and Fe+ with propane on the lowest energy (Adia-batic) potential energy surfaces. J Am Chem Soc, 1994, 116(9): 3780–3791
Wang Y C, Liu Z Y, Geng Z Y, Yang X Y. Theoretical study of activation C-O bond of CH3OCH3 by Ti+ in the gas phase. Chem Phys Lett, 2006, 427: 271–275
Lv L L, Liu X, Wang Y C, Wang H Q. DFT study of the spin-forbidden reaction between Ti+ and N2O. J Mol Struct (THEOCHEM), 2006, 774: 59–65
Gidden J, van Koppen P A M, Bowers M T. Dehydrogenation of ethene by Ti+ and V+: Excited state effects on the mechanism for C-H bond activation from kinetic energy release distributions. J Am Chem Soc, 1997, 119(17): 3935–3941
Taylor W S, May J C, Lasater A S. Reactions of Cu+ (1S, 3D) and Au+ (1S, 3D) with CH3Br. J Phys Chem A, 2003, 107(13): 2209–2215
Porembski M, Weisshaar J C. Singlet and triplet reaction paths for gas-phase Zr+C2H4 by density functional theory. J Phys Chem A, 2001, 105(20): 4851–4864
Schröder D, Shaik S, Schwarz H. Two-state reactivity as a new concept in organometallic chemistry. Acc Chem Res, 2000, 33(3): 139–145
Guo B C, Kerns K P, Jr. Castleman A W. Chemistry and kinetics of primary reactions of titanium(1+) with water, ammonia, methanol, ethane, and propene at thermal energies. J Phys Chem, 1992, 96(12): 4879–4883
Lower S K, El-Sayed M A. The triplet state and molecular electronic processes in organic molecules. Chem Rev, 1966, 66(2): 199–241
Richards W G, Trivedi H P, Cooper D L. Spin-Orbit Coupling in Molecules. New York: Oxford University Press, 1981
Shiota Y, Yoshizawa K. A spin-orbit coupling study on the spin in-version processes in the direct methane-to-methanol conversion by FeO+. J Chem Phys, 2003, 118(13): 5872–5879
Yarkony D R. Conical intersections: Diabolical and often misunder-stood. Acc Chem Res, 1998, 31(8): 511–518
Frisch M J, Trucks G W, Schlegel H B, Scuseria G E, Robb M A, Cheeseman J R, Montgomery J A, Jr. Vreven T, Kudin K N, Burant J C, Millam J M, Iyengar S S, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson G A, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox J E, Hratchian H P, Cross J B, Adamo C, Jaramillo J, Gomperts R, Stratmann R E, Yazyev O, Austin A J, Cammi R, Pomelli C, Ochterski J W, Ayala P Y, Morokuma K, Voth G A, Salvador P, Dannenberg J J, Zakrzewski V G, Dapprich S, Daniels A D, Strain M C, Farkas O, Malick D K, Rabuck A D, Raghavachari K, Foresman J B, Ortiz J V, Cui Q, Baboul A G, Clifford S, Cioslowski J, Stefanov B B, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin R L, Fox D J, Keith T, Al-Laham M A, Peng C Y, Nanayakkara A, Challacombe M, Gill P M W, Johnson B, Chen W, Wong M W, Gonzalez C, Pople J A. Gaussian 03 (Revision-B.01), Gaussian Inc., Pittsburgh PA, 2003
Yoshizawa K, Shiota Y, Yamabe T. Reaction pathway for the direct benzene hydroxylation by iron-oxo species. J Am Chem Soc, 1999, 121(1): 147–153
Lv L L, Liu X, Wang Y C, Wang H Q. A CASSCF study on photo-dissocation of the N2O3 2− dianion. Chem Phys Lett, 2006, 431: 415–420
Gonzalez C, Bernhard S H. Reaction path following in mass-weighted internal coordinates. J Phys Chem, 1990, 94(14): 5523–5527
Reed A E, Curtiss L A, Weinhold F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem Rev, 1988, 88(6): 899–926
Koseki S, Schmidt M W, Gordon M S. MCSCF/6-31G(d, p) calculations of one-electron spin-orbit coupling constants in diatomic molecules. J Phys Chem, 1992, 96(26): 10768–10772
Danovich D, Shaik S. Spin-orbit coupling in the oxidative activation of H-H by FeO+. Selection rules and reactivity effects. J Am Chem Soc, 1997, 119(7): 1773–1786
Kemper P R, Bowers M T. Electronic-state chromatography: Application to first-row transition-metal ions. J Phys Chem, 1991, 95(13): 5134–5146
Reguero M, Olivucci M, Bernardi F, Robb M A. Excited-state potential surface crossings in acrolein: A model for understanding the photochemistry and photophysics of .alpha.,.beta.-enones. J Am Chem Soc, 1994, 116(5): 2103–2114
Yoshizawa K, Shiota Y, Yamabe T. Intrinsic reaction coordinate analysis of the conversion of methane to methanol by an iron-oxo species: A study of crossing seams of potential energy surfaces. J Chem Phys, 1999, 111(2): 538–544
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by ‘Qinglan’ Talent Engineering Funds by Tianshui Normal University.
Rights and permissions
About this article
Cite this article
Lv, L., Liu, X., Yuan, K. et al. Theoretical study of the mechanism for C-H bond activation in spin-forbidden reaction between Ti+ and C2H4 . Sci. China Ser. B-Chem. 52, 295–303 (2009). https://doi.org/10.1007/s11426-009-0062-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-009-0062-7