Abstract
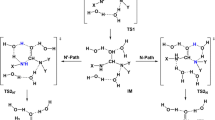
The hydrolysis mechanisms of N,N-dimethyl-N′-(2′,3′-dideoxy-3′-thiacytidine)formamidine (FA-3TC) in the gas phase and in aqueous solution were studied by use of the density functional theory B3LYP/6-31+G(d, p) method. Two possible reaction pathways in the title reaction were considered. In one pathway water attacks the C=N double bond first (path A) while in the other water attacks the C-N single bond first (path B). The calculated results indicate that the first step in both pathways is the rate-limiting process and path A is more favorable than path B in the gas phase. The effect of solvent water on the title reaction was assessed at the B3LYP/6-31+G(d, p) level of theory based on the polarizable continuum model (CPCM). In water the first mechanism (path A) is also favored.
Similar content being viewed by others
References
Anastasi C, Hantz O, Clercq F D, Pannecouque C. Potent nonclassical nucleoside antiviral drugs based on the N,N-diarylformamidine concept. J Med Chem, 2004, 47: 1183–1192
Wu Y, Xue Y, Xie D Q, Kim C K, Yan G S. Theoretical studies on the hydrolysis mechanism of N-(2-oxo-1,2-dihydro-pyrimidinyl)forma-mide. J Phys Chem B, 2007, 111: 2357–2364
Peeters D, Leroy G, Wilante C. Proton affinities and proton transfer in imine, amidine and guanidine series. J Mol Struct, 1997, 416: 21–32
Simperler A, Mikenda W, Schwarz K. Proton motion and proton transfer in the formamidine-formic acid complex: An ab initio projector augmented wave molecular dynamics study. Chem Eur J, 2001, 7: 1606–1613
Zhang Q, Bell R, Truong T N. Ab Initio and density functional theory studies of proton transfer reactions in multiple hydrogen bond systems. J Phys Chem, 1995, 99: 592–599
Nguyen K A, Gordon M S, Truhlar D G. Effect of hydration and dimerization of the formamidine rearrangement. J Am Chem Soc, 1991, 113: 1596–1600
Lim J H, Lee E K, Kim Y. Theoretical study for solvent effect on the potential energy surface for the double proton transfer in formic acid dimer and formamidine dimer. J Phys Chem, 1997, 101: 2233–2239
Andrés J, Beltran A, Carda M, Krechl J, Monterde J, Silla E. Amidine decomposition mechanism. A theoretical study. J Mol Struct (Theochem), 1992, 254: 465–472
Flinn C, Poirier R A, Sokalski W A. Ab initio study of the deamination of formamidine. J Phys Chem A, 2003, 107: 11174–11181
Fukui K. A formulation of the reaction coordinate. J Phys Chem, 1970, 74: 4161–4163
Barone V, Cossi M. Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J Phys Chem A, 1998, 102: 1995–2001
Frisch M J, Trucks G W, Schlegel H B, Scuseria G E, Robb M A, Cheeseman J R, Montgomery Jr. J A, Vreven T, Kudin K N, Burant J C, Millam J M, Iyengar S S, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson G A, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox J E, Hratchian H P, Cross J B, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann R E, Yazyev O, Austin A J, Cammi R, Pomelli C, Ochterski J W, Ayala P Y, Morokuma K, Voth G A, Salvador P, Dannenberg J J, Zakrzewski V G, Dapprich S, Daniels A D, Strain M C, Farkas O, Malick D K, Rabuck A D, Raghavachari K, Foresman J B, Ortiz J V, Cui Q, Baboul A G, Clifford S, Cioslowski J, Stefanov B B, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin R L, Fox D J, Keith T, Al-Laham M A, Peng C Y, Nanayakkara A, Challacombe M, Gill P M W, Johnson B, Chen W, Wong M W, Gonzalez C, Pople J A. Gaussian 03. Version D01. Gaussian, Inc., Wallingford CT, 2005
Reed A E, Curtiss L A, Weinhold F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem Rev, 1988, 88: 899–926
Xue Y, Kang C H, Kim C K, Lee I. Theoretical studies on the gas-phase pyrolysis of 2-phenoxycarboxylic acids: an ONIOM approach. J Comput Chem, 2003, 24: 963–972
Xue Y, Kim C K, Guo Y, Xie D Q, Yan G S. DFT study and Monte Carlo simulation on proton transfers of 2-amino-2-oxazoline, 2-amino-2-thiazoline, and 2-amino-2-imidazoline in the gas phase and in water. J Comput Chem, 2005, 26: 994–1005
Moyano A, Pericàs M A, Valent A. A theoretical study on the mechanism of the thermal and the acid-catalyzed decarboxylation of 2-oxetanones (β-lactones). J Org Chem, 1989, 54: 573–582
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Natural Science Foundation of China (Grant Nos. 20473055 and 20773089) and the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry (Grant No. 20071108-18-15)
Rights and permissions
About this article
Cite this article
Zhang, C., Xue, Y. Theoretical study on the hydrolysis mechanism of N,N-dimethyl-N′-(2′,3′-dideoxy-3′-thiacytidine)formamidine. Sci. China Ser. B-Chem. 51, 911–917 (2008). https://doi.org/10.1007/s11426-008-0047-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-008-0047-y