Abstract
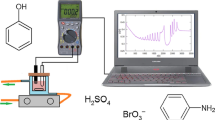
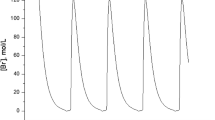
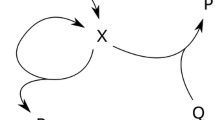
The ferroin-catalyzed Belousov-Zhabotinsky (BZ) reaction, the oxidation of malonic acid by acidic bromate, is the most commonly investigated chemical system for understanding spatial pattern formation. Various oscillatory behaviors were found from such as mixed-mode and simple period-doubling oscillations and chaos on both Pt electrode and Br-ISE at high flow rates to mixed-mode oscillations on Br-ISE only at low flow rates. The complex dynamic behaviors were qualitatively reproduced with a two-cycle coupling model proposed initially by Györgyi and Field. This investigation offered a proper medium for studying pattern formation under complex temporal dynamics. In addition, it also shows that complex oscillations and chaos in the BZ reaction can be extended to other bromate-driven nonlinear reaction systems with different metal catalysts.
Similar content being viewed by others
References
Zhabotinskii A M. Periodic kinetics of oxidation of malonic acid in solution (study of the Belousov reaction kinetics). Biofizika, 1964, 9: 306–311
Zhabotinshii A M. Periodic liquid-phase oxidation reactions. Dokl Akad Nauk SSSR, 1964, 157: 392–395
Field R J, Körös E, Noyes R M. Oscillations in chemical systems. II. Thorough analysis of temporal oscillation in the bromatecerium-malonic acid system. J Am Chem Soc, 1972, 94: 8649–8664
Field R J, Burger M. Oscillations and Traveling Waves in Chemical Systems. New York: Wiley, 1985
Rouff P, Noyes R M. An amplified Oregonator model simulating alternative excitabilities, transitions in types of oscillations and temporary bistability in a closed system. J Chem Phys, 1986, 84: 1413–1423
Noszticzius Z, McCormick W D, Swinney H L. Use of bifurcation diagrams as fingerprints of chemical mechanisms. J Phys Chem-US, 1989, 93: 2796–2800
Gyorgyi L, Field R J, Noszticzius Z, McCormick W D, Swinney H L. Confirmation of high flow rate chaos in the Belousov-Zhabotinskii reaction. J Phys Chem-US, 1992, 96: 1228–1233
Nagygyory S, Wittmann M, Pintér S, Visegrády A, Dancsó A, Thuy N B, Noszticzius Z. Alternative reaction channels and carbene intermediates in the Ce4+-malonic acid and Ce4+-bromomalonic acid reactions. 1. CO2 measurements. J Phys Chem A, 1999, 103: 4885–4892
De kepper P, Rossi A, Pacault A. Etude d’une réaction chimique périodique. Diagramme d’État de la réaction de Belousov-Zhabotinskii. C R Acad Sci Ser C, 1976, 283: 371–375
De kepper P, Bar-Eli K. Dynamical properties of the Belousov-Zhabotinskii reaction in a flow system: Theoretical and experimental analysis. J Phys Chem-US, 1983, 87: 480–488
Ruoff P, Noyes R M. Chemical oscillations and instabilities; Part 61. Temporary bistability and unusual oscillatory behavior in a closed Belousov-Zhabotinskii reaction system. J Phys Chem-US, 1985, 89: 1339–1341
Maselko J, Swinney H L. Complex periodic oscillations and Farey arithmetic in the Belousov-Zhabotinskii reaction. J Chem Phys, 1986, 85: 6430–6441
Bergé P, Pomeau Y, Vidal C. Order within Chaos. New York: Wiley, 1986
Strizhak P E, Kawczyńskim A L. Complex transient oscillations in the Belousov-Zhabotinskii reaction in a batch reactor. J Phys Chem-US, 1995, 99: 10830–10833
Wang J, Zhao J, Chen Y, Gao Q, Wang Y. Coexistence of two bifurcation regimes in a closed ferroin-catalyzed Belousov-Zhabotinsky reaction. J Phys Chem A, 2005, 109: 1374–1381
Rachwalska M, Kawczyński A L. Regularities in complex transient oscillations in the Belousov-Zhabotinsky reaction in a CSTR. J Phys Chem A, 1997, 101: 1518–1522
Kalishin E Yu, Goncharenko M M, Khavrus V A, Strizhak V A. Periodic, mixed-mode, and chaotic regimes in the Belousov-Zhabotinskii reaction catalyzed by ferroin. Kinet Catal, 2002, 43: 233–244
Moss F. Noisy waves. Nature, 1998, 391: 743–744
Vanag V K, Epstein I R. Inwardly rotating spiral waves in a reaction-diffusion system. Science, 2001, 294: 835–837
Park J S, Woo S, Lee K. Transverse instability of line defects of period-2 spiral waves. Phys Rev Lett, 2004, 93: 098302-2–098302-4
Wolf A, Swift J B, Swinney H L, Vastano J A. Determining Lyapunov exponents from a time series. Physica D, 1985, 16: 285–317
Györgyi L, Field R J. Simple models of deterministic chaos in the Belousov-Zhabotinskii reaction. J Phys Chem-US, 1991, 95: 6594–6602
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Natural Science Foundation of China (Grant No. 20573134) and the Program for New Century Excellent Talents in Chinese University (Grant No. NCET-05-0477)
Rights and permissions
About this article
Cite this article
Zong, C., Gao, Q., Wang, Y. et al. Period-doubling and chaotic oscillations in the ferroin-catalyzed Belousov-Zhabotinsky reaction in a CSTR. SCI CHINA SER B 50, 205–211 (2007). https://doi.org/10.1007/s11426-007-0026-8
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11426-007-0026-8