Abstract
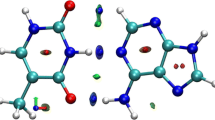
Density functional theory B3LYP is employed to obtain the optimized geometries of the ground state and interaction energy for triazines and water complexes. The results show that the 1,2,3-triazine-water, 1,2,4-triazine-water and 1,3,5-triazine-water complex on the ground state have Cs, Cs and C1 symmetry, and strong hydrogen bonding interaction with −17.83, −17.38 and −13.55 kJ/mol after basis set superposition error and zero-point vibration energy correction, respectively, and large red-shift for the symmetric H-O stretching vibration frequencies forming N...H-O hydrogen bond in the triazines complex. The first singlet (n, π*) vertical excitation energy of the monomer and the hydrogen bonding complexes between triazines and water is investigated by time-dependent density functional theory.
Similar content being viewed by others
References
Rablen P R, Lockman J W, Jorgensen W L. The hydrogen bond between water and aromatic bases of biological interest: Rotational spectrum of pyridazine-water. J Phys Chem A, 1998, 102: 8097–8100
Cai Z L, Reimers J R. The first singlet (n, π*) and (π, π*) excited states of the hydrogen-bonded complex between water and pyridine. J Phys Chem A, 2002, 106: 8769–8778
Zhang B, Cai Y, Mu X L, et al. Multiphoton ionization and density functional studies of pyrimidine-(water)n clusters. J Chem Phys, 2002, 117: 3701–3710
Zhang B, Cai Y, Mu X L, et al. Multiphoton ionization and ab initio calculation studies of pyridazine-(water)n clusters. Chem Phys, 2002, 276: 277–292
Navarro A, Vázquez J, Montejo M, et al. A reinvestigation of the v7 and v10 modes of pyridazine on the basis of the inelastic neutron scattering spectrum analysis. Chem Phys Lett, 2002, 361: 483–491
Xie D Q, Ma X H, Zeng J. Medium effects on the lowest 1(n, π*) excitation of 1,2,3-triazine in water. Chem Phys Lett, 2003, 368: 377–383
Palmer M H, McNab H, Walker I C, et al. The electronic states and molecular properties of 1,2,3-triazine. Chem Phys, 1998, 228: 39–59
Fischer G, Smith D M, Nwankwoala A U. The electronic spectroscopy of 1,2,3-triazine. Chem Phys, 1997, 221: 11–21
Palmer M H, Walker I C, Guest M F, Siggel M R F. Chem Phys, 1995, 201: 381–391
Walker I C, Palmer M H, Ballard C C. The electronic states of azines. Chem Phys, 1992, 167: 61–75
Zeng J, Xie D Q. Hydrogen bonding and solvent effects on the lowest 1(n, π*) excitations of triazines in water. J Comput Chem, 2004, 25: 813–822
Frish M J, Trucks G W, Schlegel H B, et al. Gaussian 98, Revision A9. Pittsburgh: Gaussian Inc., 1998
Dkhissi A, Adamowicz L, Maes G. Density functional theory study of the hydrogen-bonded pyridine-H2O complex. J Phys Chem A, 2000, 104: 2112–2119
Zeng J, Craw J S, Hush N S, et al. Medium effects on molecular and ionic electronic spectra application to the lowest 1(n, π*) state of dilute pyridine in water. Chem Phys Lett, 1993, 2006: 323–328
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Li, Q. Structure and property of the hydrogen bonding complex between triazines and water. SCI CHINA SER B 49, 209–213 (2006). https://doi.org/10.1007/s11426-006-0209-8
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11426-006-0209-8