Abstract
Background
Hip hemiarthroplasty is a well-established treatment of displaced femoral neck fracture, although debate exists over whether cemented or uncemented fixation is superior. Uncemented prostheses have typically been used in younger, healthier patients and cemented prostheses in older patients with less-stable bone. Also, earlier research has suggested that bone cement has cytotoxic effects and may trigger cardiovascular and respiratory adverse events.
Questions/Purposes
The aim of this systematic review and meta-analysis was to compare morbidity and mortality rates after cemented and uncemented hemiarthroplasty for the treatment of displaced femoral neck fractures in elderly patients.
Methods
Using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, we searched seven medical databases for randomized clinical trials and observational studies. We compared cemented and uncemented hemiarthroplasty using the Harris Hip Score (HHS), as well as measures of postoperative pain, mortality, and complications. Data were extracted and pooled as risk ratios or standardized mean difference with their corresponding 95% confidence intervals in a meta-analysis model.
Results
The meta-analysis included 34 studies (12 randomized trials and 22 observational studies), with a total of 42,411 patients. In the pooled estimate, cemented hemiarthroplasty was associated with less risk of postoperative pain than uncemented hemiarthroplasty. There were no significant differences between groups regarding HHS or rates of postoperative mortality, pulmonary embolism, cardiac arrest, myocardial infarction, acute cardiac arrhythmia, or deep venous thrombosis.
Conclusions
While we found that cemented hemiarthroplasty results in less postoperative pain than uncemented hemiarthroplasty in older patients with femoral neck fracture, the lack of significant differences in functional hip scores, mortality, and complications was surprising. Further high-level research is needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
CME Program Description
This HSS Journal® continuing medical education (CME) activity has been developed by the journal editors. After completing this activity, learners will be able to demonstrate an increase in or affirmation of their knowledge of the relevant topic. They will also be able to evaluate the appropriateness of clinical data and apply it to their practice and the provision of patient care.
Program Format
In each edition, the HSS Journal will contain one clinically relevant article selected by the editors to be designated for CME credit.
Accreditation
Hospital for Special Surgery is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide CME for physicians.
Hospital for Special Surgery designates this journalbased CME activity for a maximum of 1.0 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Disclosure Information
None of the individuals in a position to control the content of this CME activity have any relevant financial relationships to disclose.
Completion Requirement
To complete the activity and earn credit, the participant must read the article, complete an online knowledge assessment with a score of 100%, and complete the evaluation.
To complete the activity and earn CME credit
Go to https://bit.ly/HSSJournalCME. Please note that first-time users will need to create a free HSS eAcademy® account to access the course page.
Register for the activity, proceed through the article, and complete the post-course knowledge assessment.
Once you have completed the knowledge assessment with a score of 100%, you will be prompted to complete an evaluation and generate your certificate of CME credit.
If you have any questions, please email professionaleducation@hss.edu or phone 212-606-1057.
Introduction
Displaced femoral neck fractures are associated with persistent hip pain, disability, and high morbidity and mortality rates, significantly affecting quality of life [4, 5, 18]. Debate continues over the selection of prosthesis to be used for hemiarthroplasty [3, 28, 40, 43, 54, 55, 56].
Historically, the use of uncemented femoral components has been indicated in younger-elderly patients with relatively good bone quality, although disadvantages include higher risks of thigh pain and periprosthetic fracture [27, 40]. Cemented femoral components are typically used in elderly patients with poor bone quality and are associated with less thigh pain and stem loosening [53], but they have been associated with higher risks of cardiac events, deep venous thrombosis (DVT), and pulmonary embolism as a result of bone cement implantation syndrome [1, 19, 3839, 40, 65]. Various studies have reported that bone cement can have cytotoxic effects and mediate procoagulant activities, which could trigger cardiovascular and respiratory events, the main causes of death in elderly patients with reduced reserve capacity [14, 15, 19].
Consequently, we conducted a systematic review and meta-analysis to compare the rates of mortality and complications, including pulmonary embolism, cardiac arrest, myocardial infarction, acute cardiac arrhythmia, and DVT, after cemented and uncemented hemiarthroplasty used for the treatment of displaced femoral neck fractures in older patients.
Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (www.prisma-statement.org) as our guide during the preparation of this systematic review and meta-analysis. Moreover, all steps were performed in strict accordance with the Cochrane Handbook of Systematic Reviews of Interventions [33].
We performed electronic searches of PubMed, the Cochrane Central Register of Controlled Trials (CENTRAL), Scopus, Embase, EBSCO, Ovid, and Web of Science in May of 2017, using the following keywords: “hemiarthroplasty,” “arthroplasty,” “femoral neck fractures,” “intracapsular hip fractures,” “hip prosthesis,” “cemented,” “cementless,” and “uncemented.” We modified terms as necessary to suit each database and applied no restrictions of publication date. We also searched the US clinical trial registry (www.clinicaltrials.gov) for additional ongoing and unpublished studies and searched the reference lists in eligible studies for relevant articles not otherwise identified.
We included randomized clinical trials and observational studies that met the following inclusion criteria: the study enrolled patients over 65 years who underwent surgery for displaced femoral neck fractures, the intervention was hemiarthroplasty with a cemented or uncemented (cementless) prosthesis, and the study compared the outcomes of cemented and uncemented hemiarthroplasty.
We excluded reviews, case reports, and duplicates, as well as studies in which patients had had a previous fracture of the same hip or a pathological fracture, in which an animal model was used, or that were not in English. Eligibility screening was conducted in two steps, each by three independent reviewers: title and abstract screening for matching the inclusion criteria and full-text screening to determine eligibility for meta-analysis. Disagreements were resolved by a third reviewer.
The outcomes of interest included hip function as assessed by the Harris Hip Score (HHS) [31, 50], postoperative pain, medical outcomes (including pulmonary embolism, cardiac arrest, myocardial infarction, acute cardiac arrhythmia, and DVT), and mortality rates at 1 month, 3 months, and 1 year after surgery.
Data were extracted from the included studies by three independent researchers using Microsoft Excel. Disagreements were resolved by discussion and consensus among senior researchers. Extracted data included first author, publication year, study design, number of participants in each group, mean age, sex, type of intervention, study period, follow-up period, and outcomes of interest. For the randomized clinical trials, we used the Cochrane Collaboration’s tool for assessing the risk of bias [33]. For observational studies, we used the Newcastle–Ottawa Scale for assessing the quality of observational studies [66], and each included study was assessed according to reporting of three essential domains: selection of the study subjects; comparability of groups, in terms of demographic characteristics and important potential confounders; and ascertainment of the prespecified outcome (exposure/treatment). To assess the risk of bias across the included studies, we compared the reported outcomes between all studies to exclude selective reporting of outcomes.
Data Analysis
We calculated risk ratios (RRs) with 95% confidence intervals (CIs) for dichotomous outcomes and standardized mean difference (SMD) with 95% CI for continuous data. Heterogeneity was assessed using the Cochran Q test, χ2 test for Q statistic distribution, and the I2 test. We performed the meta-analysis using a fixed-effect model if no significant heterogeneity was present (I2 < 50%; p > 0.1). Otherwise, we adopted the random-effect model. Egger’s test and the trim-and-fill method were used to assess the possibility of publication bias. Data analyses were performed using the R software “meta” package, version 4.9–2 (R Foundation, Vienna, Austria), for Windows. A p value of < 0.05 was considered statistically significant.
Results
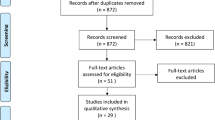
The literature search yielded 871 unique records. Upon screening of titles and abstracts, 50 articles were retrieved and screened for eligibility. Of these, 34 articles were included in the meta-analysis. The study selection process is shown in the PRISMA flow diagram (Fig. 1).
Of the 34 studies included in our analysis, 12 were randomized clinical trials [17, 20, 22, 25, 42, 55, 59, 60, 65, 68, 69, 73] and 22 were observational studies [2, 7, 9, 10, 24, 27, 29, 30, 35, 37, 40, 44, 47, 51, 52, 57, 61, 70, 72, 75,76,77]. The 34 included studies investigated a total of 42,411 participants; among whom 32,385 underwent cemented hemiarthroplasty and 10,026 underwent uncemented hemiarthroplasty (Table 1). The risk of bias in the randomized clinical trials was acceptable according to the Cochrane risk-of-bias assessment (Fig. 2a). The observational studies achieved a mean of 7 out of 9 points on the Newcastle–Ottawa Scale, indicating a moderate quality (Fig. 2b).
When the HHS was assessed (five studies: three randomized clinical trials and two observational studies), the overall estimate showed no significant difference between the cemented and uncemented hemiarthroplasty groups (SDM = 0.08; 95% CI, − 0.22, to 0.37; p = 0.81). This effect estimate was consistent in subgroup analyses (Fig. 3) at follow-up times of 3 months (SMD = 0.28; 95% CI, − 0.33 to 0.89; p = 0.23), 1 year (SMD = 0.07; 95% CI, − 0.40 to 0.53; p = 0.66), and 5 years (SMD = − 0.19; 95% CI, − 0.92 to 0.54; p = 0.17).
The cemented hemiarthroplasty group was found to have a lower risk of postoperative pain. Eleven studies (seven randomized clinical trials and four observational studies) reported on postoperative pain (overall RR = 0.64; CI, 0.53 to 0.77; p < 0.0001). This effect estimate remained consistent in subgroup analyses according to study design (Fig. 4); no significant heterogeneity was observed (I2 = 25%; p = 0.21).
No significant differences in mortality were found between the cemented and uncemented hemiarthroplasty groups at any duration of follow-up.
Nine studies (four randomized clinical trials and five observational studies) reported on mortality at 1 month postoperatively. There was no significant difference between the two groups (RR = 0.86; 95% CI, 0.61 to 1.21; p = 0.39); there was moderate heterogeneity (I2 = 36%; p = 0.32). This result remained consistent in subgroup analysis according to study design (Fig. 5a).
a Forest plot showing the risk ratio (RR) of mortality at 1 month postoperatively between cemented and uncemented hemiarthroplasty (with 95% confidence interval [CI]); b forest plot showing the RR of mortality at 3 months postoperatively between cemented and uncemented hemiarthroplasty (with 95% CI); c forest plot showing the RR of mortality at 1 year postoperatively between cemented and uncemented hemiarthroplasty (with 95% CI)
Six studies (four randomized clinical trials and two observational studies) reported on mortality at 3 months postoperatively. The overall pooled RR did not favor either of the two groups (RR = 0.87; 95% CI, 0.66 to 1.14; p = 0.31); there was no evidence of heterogeneity (I2 = 0%; p = 0.69). This result remained consistent in subgroup analysis according to study design (Fig. 5b).
Data on mortality at 1 year postoperatively were reported in 13 studies (eight randomized clinical trials and five observational studies), with no significant difference between cemented and uncemented hemiarthroplasty reported (RR = 0.92; 95% CI, 0.80 to 1.06; p = 0.25) and no evidence of heterogeneity (I2 = 0%; p = 0.82). This result remained consistent in subgroup analysis according to study design (Fig. 5c). Egger’s test showed no evidence of publication bias; p = 0.31.
No difference in the rates of pulmonary embolism or DVT was found. Eight studies (two randomized clinical trials and six observational studies) reported data on pulmonary embolism. The overall RR did not favor either prosthesis type (RR = 1.13; 95% CI, 0.64 to 2.02; p = 0.67). This result remained true regardless of study design. Pooled studies were homogeneous (I2 = 0%; p = 0.70) (Fig. 6a). Eight studies reported data on DVT (one randomized clinical trial and seven observational studies). The overall RR did not differ significantly between the two groups (RR = 0.85; 95% CI, 0.50 to 1.44; p =0.54); there was no notable heterogeneity among these studies (I2 = 14%; p = 0.32) (Fig. 6c).
a Forest plot showing the risk ratio (RR) of pulmonary embolism between cemented and uncemented hemiarthroplasty (with 95% confidence interval [CI]); b forest plot showing the RR of myocardial infarction between cemented and uncemented hemiarthroplasty (with 95% CI); c forest plot showing the RR of deep venous thrombosis between cemented and uncemented hemiarthroplasty (with 95% CI)
No difference in the risk of cardiac complications was found between the two groups. Two studies (one randomized clinical trial and one observational study) reported on cardiac arrest. The combined RR did not favor either of the two groups (RR = 1.74; 95% CI, 0.13 to 23.19; p = 0.68). Pooled studies were heterogeneous (I2 = 60%; p = 0.67). Similarly, the overall RR in the eight studies reporting data on myocardial infarction (three randomized clinical trials and five observational studies) was comparable between the two groups (RR = 1.44; 95% CI, 0.73 to 2.86; p = 0.30). This result remained consistent in subgroup analysis according to study design. The eight pooled studies were homogeneous (I2 = 0%; p = 0.84) (Fig. 6b). Two studies (one randomized clinical trial and one observational study) provided data on acute cardiac arrhythmia. The combined RR did not favor either cemented or uncemented hemiarthroplasty (RR = 0.57; 95% CI, 0.08 to 4.33; p = 0.59). This effect estimate remained consistent in subgroup analysis according to study design. No heterogeneity was observed (I2 = 0%; p = 0.64).
Discussion
This study of 42,411 older adults showed no significant differences between cemented and uncemented hemiarthroplasty of the hip in terms of HHS, mortality, or medical complications. However, the results did reveal cemented fixation to be associated with less postoperative pain than uncemented fixation.
Similar to our results, those of a study from the Swedish Hip Arthroplasty Register by Rogmark et al. showed no significant difference in mortality according to femoral fixation type at 1 year after surgery [58]. In addition, two recent meta-analyses, one with five randomized clinical trials [71] and the other with seven [46], reported no significant differences in mortality related to type of fixation (cemented or uncemented) 1 year after surgery. In contrast, a study of data from an Australian registry reported higher mortality on the first postoperative day in patients with cemented prostheses but an overall lower rate of death through 1 year of follow-up [12].
Several studies have described comorbidity, older age, male sex, delayed surgery, and cognitive impairment as some of the most important risk factors for death after hip fracture [34, 36, 56, 6263, 6768]. Our patient population may lack the reserve capacity that is essential to handle the double trauma of a hip fracture and subsequent surgery. Therefore, the more severe the comorbidity, the higher the risk of a fatal outcome when cementation is applied; these factors, of course, influence the selection of the method of fixation [68]. Nevertheless, recent improvements in surgical techniques, the careful elimination of any cellular debris and blood clots from the medullary canal before inserting the bone cement, perioperative monitoring of patients by an experienced anesthesia team, and thromboprophylaxis may well have improved the survival of hip surgery patients [21, 26, 32, 43, 64, 69] and help explain our results regarding mortality at 1 month, 3 months, and 1 year after surgery.
Earlier research has suggested that cemented femoral components in hip replacement surgery in patients with femoral neck fractures are associated with cardiovascular complications, including embolism and arrhythmia [22, 23, 45, 48, 65]. Nevertheless, our study found no differences between cemented and uncemented hemiarthroplasty in rates of postoperative myocardial infarction, acute arrhythmia, cardiac arrest, or pulmonary embolism. Our findings are supported by meta-analyses conducted by Li et al., Lin et al., and Luo et al., which found no differences in major postoperative complications between patients with cemented and cementless stems [46, 47, 49].
High risks of DVT after cemented hip and knee arthroplasty have been reported in earlier studies [13, 41, 49]. The hypercoagulable state that follows femoral neck fracture is associated with an increased risk of thromboembolism, and factors enhancing hypercoagulability include, in addition to the initial trauma, subsequent surgery, blood loss resulting from either fracture or surgery, and perioperative fluid therapy [74]. Furthermore, the thrombogenic properties of the bone cement contribute to the pathogenesis of DVT. Polymethylmethacrylate monomer found in mixed venous blood during cemented arthroplasty induces secretion of platelet activation factors, such as transforming growth factor β and β-thromboglobulin, and stimulates monocytes to express tissue factor, which triggers coagulation [6, 16]. Additionally, higher levels of cytokine CD14/42a, a known measure of platelet–monocyte aggregation, are present in patients undergoing cemented arthroplasty [8]. In contrast, a small study by Hong et al. reported no statistically significant difference in DVT development between cemented and uncemented hemiarthroplasty prostheses used to treat traumatic displaced femoral neck fractures (3.0% [n = 4] and 5.1% [n = 7], respectively) [35].
Clarke et al. studied the bone cement as a risk factor for DVT, comparing three sets of patients undergoing a cemented or uncemented total knee replacement (TKR) or a cemented total hip replacement (THR) [11]. They found that uncemented prostheses were associated with a greater prevalence of DVT at 5 to 7 days than cemented prostheses, and both knee groups had a significantly higher prevalence of DVT than the cemented THR group. The thrombi were significantly longer after cemented TKR (26.5 cm) than after both uncemented TKR (11 cm) and cemented THR (7 cm). The authors concluded that the bone cement may influence the length of a thrombus but does not lead to an increase in the incidence of DVT.
Some study limitations exist. It is possible that unbalanced cohort sizes and the use of different types of hip prosthesis limit the study’s power to detect differences between cohorts. Additionally, comorbidity as a risk factor for death was not well assessed in all of the included studies. Another limitation is the inclusion of only English-language literature; relevant studies in other languages might have been omitted. Finally, causality cannot be determined in observational studies, limiting the conclusions of our meta-analysis.
In conclusion, current evidence shows patients treated with cemented hemiarthroplasty experience less postoperative pain than those treated with uncemented prosthesis. Our meta-analysis shows no significant differences between cemented and uncemented hip hemiarthroplasty in terms of functional hip score (the HHS); postoperative mortality; or medical complications, including pulmonary embolism, cardiac arrest, myocardial infarction, acute cardiac arrhythmia, and DVT. The absence of a connection between cemented prostheses and complications is surprising, considering earlier research findings and the use of cemented femoral components historically in older patients with poor bone quality and greater comorbidity. As surgical techniques and perioperative care continue to improve, further research should be conducted to confirm our findings.
References
Baker RP, Squires B, Gargan MF, Bannister GC. Total hip arthroplasty and hemiarthroplasty in mobile, independent patients with a displaced intracapsular fracture of the femoral neck. A randomized, controlled trial. J Bone Jt Surg Am. 2006;88:2583–2589. https://doi.org/10.2106/JBJS.E.01373.
Bell KR, Clement ND, Jenkins PJ, Keating JF. A comparison of the use of uncemented hydroxyapatite-coated bipolar and cemented femoral stems in the treatment of femoral neck fractures: a case-control study. Bone Jt J. 2014;96-b:299–305. https://doi.org/10.1302/0301-620x.96b3.32271.
Bezwada HP, Shah AR, Harding SH, Baker J, Johanson NA, Mont MA. Cementless bipolar hemiarthroplasty for displaced femoral neck fractures in the elderly. J Arthroplast. 2004;19:73–77.
Bhandari M, Devereaux PJ, Tornetta 3rd P, et al. Operative management of displaced femoral neck fractures in elderly patients. An international survey. J Bone Jt Surg Am. 2005;87:2122–2130. https://doi.org/10.2106/jbjs.E.00535.
Branco JC, Felicissimo P, Monteiro J. Epidemiology of hip fractures and its social and economic impact. A revision of severe osteoporosis current standard of care.. Acta Reum Port. 2009;34:475–485.
Cenni E, Granchi D, Vancini M, Pizzoferrato A. Platelet release of transforming growth factor-beta and beta-thromboglobulin after in vitro contact with acrylic bone cements. Biomaterials. 2002;23:1479–1484.
Chana R, Mansouri R, Jack C, Edwards MR, Singh R, Keller C, et al. The suitability of an uncemented hydroxyapatite coated (HAC) hip hemiarthroplasty stem for intra-capsular femoral neck fractures in osteoporotic elderly patients: the Metaphyseal-Diaphyseal Index, a solution to preventing intra-operative periprosthetic fr. J Orthop Surg Res 2011;6:59. https://doi.org/10.1186/1749-799x-6-59.
Cheng K, Giebaly D, Campbell A, Rumley A, Lowe G. Systemic effects of polymethylmethycrylate in total knee replacement: A prospective case-control study. Bone Jt Res. 2014;3:108–116. https://doi.org/10.1302/2046-3758.34.2000230.
Choi JY, Sung YB, Kim JH. Comparative study of bipolar hemiarthroplasty for femur neck fractures treated with cemented versus cementless stem. Hip Pelvis. 2016;28:208–216. https://doi.org/10.5371/hp.2016.28.4.208.
Cicek H, Seyfettinoglu F, Kilicarslan K, Ogur HU, Ozturk L, Inkaya E. What should be the preferred choice of hemiarthroplasty technique in American Society of Anesthesiologists (ASA) class III patients with femoral neck fractures? Cemented or cementless. Injury. 2015;46:1567–1570. https://doi.org/10.1016/j.injury.2015.05.019.
Clarke MT, Green JS, Harper WM, Gregg PJ. Cement as a risk factor for deep-vein thrombosis. Comparison of cemented TKR, uncemented TKR and cemented THR. J Bone Jt Surg Br. 1998;80:611–613.
Costain DJ, Whitehouse SL, Pratt NL, Graves SE, Ryan P, Crawford RW. Perioperative mortality after hemiarthroplasty related to fixation method. Acta Orthop. 2011;82:275–281. https://doi.org/10.3109/17453674.2011.584208.
Dahl OE, Molnar I, Ro JS, Vinje A. Global tests on coagulation and fibrinolysis in systemic and pulmonary circulation accompanying hip arthroplasty with acrylic cement. Thromb Res. 1988;50:865–873.
Dahl OE, Johnsen H, Kierulf P, Molnar I, Ro JS, Vinje A, et al. Intrapulmonary thrombin generation and its relation to monomethylmethacrylate plasma levels during hip arthroplasty. Acta Anaesthesiol Scand. 1992;36:331–335.
Dahl OE, Garvik LJ, Lyberg T. Toxic effects of methylmethacrylate monomer on leukocytes and endothelial cells in vitro. Acta Orthop Scand. 1994;65:147–153.
Dahl OE, Westvik AB, Kierulf P, Lyberg T. Effect of monomethylmethacrylate on procoagulant activities of human monocytes and umbilical vein endothelial cells in vitro. Thromb Res. 1994;74:377–387.
Deangelis JP, Ademi A, Staff I, Lewis CG. Cemented versus uncemented hemiarthroplasty for displaced femoral neck fractures: a prospective randomized trial with early follow-up. J Orthop Trauma. 2012;26:135–140. https://doi.org/10.1097/BOT.0b013e318238b7a5.
Dhanwal DK, Dennison EM, Harvey NC, Cooper C. Epidemiology of hip fracture: Worldwide geographic variation. Indian J Orthop. 2011;45:15–22. https://doi.org/10.4103/0019-5413.73656.
Donaldson MG, Palermo L, Ensrud KE, Hochberg MC, Schousboe JT, Cummings SR. Effect of alendronate for reducing fracture by FRAX score and femoral neck bone mineral density: the Fracture Intervention Trial. J Bone Min Res. 2012;27:1804–1810. https://doi.org/10.1002/jbmr.1625.
Dorr LD, Glousman R, Hoy AL, Vanis R, Chandler R. Treatment of femoral neck fractures with total hip replacement versus cemented and noncemented hemiarthroplasty. J Arthroplast. 1986;1:21–8.
Eiskjaer S, Ostgard SE. Risk factors influencing mortality after bipolar hemiarthroplasty in the treatment of fracture of the femoral neck. Clin Orthop Relat Res. 1991:295–300.
Emery RJ, Broughton NS, Desai K, Bulstrode CJ, Thomas TL. Bipolar hemiarthroplasty for subcapital fracture of the femoral neck. A prospective randomised trial of cemented Thompson and uncemented Moore stems. J Bone Jt Surg Br. 1991;73:322–324.
Fallon KM, Fuller JG, Morley-Forster P. Fat embolization and fatal cardiac arrest during hip arthroplasty with methylmethacrylate. Can J Anaesth. 2001;48:626–629. https://doi.org/10.1007/BF03016194.
Faraj AA, Branfoot T. Cemented versus uncemented Thompson’s prostheses: a functional outcome study. Injury. 1999;30:671–675. https://doi.org/10.1016/s0020-1383(99)00169-2.
Figved W, Opland V, Frihagen F, Jervidalo T, Madsen JE, Nordsletten L. Cemented versus uncemented hemiarthroplasty for displaced femoral neck fractures. Clin Orthop Relat Res. 2009;467:2426–2435. https://doi.org/10.1007/s11999-008-0672-y.
Fisher AA, Davis MW, Rubenach SE, Sivakumaran S, Smith PN, Budge MM. Outcomes for older patients with hip fractures: the impact of orthopedic and geriatric medicine cocare. J Orthop Trauma. 2006;20:172–180. https://doi.org/10.1097/01.bot.0000202220.88855.16.
Foster AP, Thompson NW, Wong J, Charlwood AP. Periprosthetic femoral fractures--a comparison between cemented and uncemented hemiarthroplasties. Injury. 2005;36:424–429. https://doi.org/10.1016/j.injury.2004.07.023.
Frihagen F, Madsen JE, Aksnes E, et al. Comparison of re-operation rates following primary and secondary hemiarthroplasty of the hip. Injury. 2007;38:815–819. https://doi.org/10.1016/j.injury.2006.09.020.
Gjertsen JE, Lie SA, Vinje T, et al. More re-operations after uncemented than cemented hemiarthroplasty used in the treatment of displaced fractures of the femoral neck: an observational study of 11,116 hemiarthroplasties from a national register. J Bone Jt Surg Br. 2012;94:1113–1119. https://doi.org/10.1302/0301-620x.94b8.29155.
Grammatopoulos G, Wilson HA, Kendrick BJ, et al. Hemiarthroplasty using cemented or uncemented stems of proven design: a comparative study. Bone Jt J. 2015;97-b:94–99. https://doi.org/10.1302/0301-620x.97b1.34138.
Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. J Bone Jt Surg Am. 1969;51(4):737–755.
Heidari N, Jehan S, Alazzawi S, Bynoth S, Bottle A, Loeffler M. Mortality and morbidity following hip fractures related to hospital thromboprophylaxis policy. Hip Int. 2012;22:13–21. https://doi.org/10.5301/HIP.2012.9079.
Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. Vol. 4. John Wiley & Sons; 2011.
Holvik K, Ranhoff AH, Martinsen MI, Solheim LF. Predictors of mortality in older hip fracture inpatients admitted to an orthogeriatric unit in oslo, norway. J Aging Heal. 2010;22:1114–1131. https://doi.org/10.1177/0898264310378040.
Hong CC, Nashi N, Makandura MC, Krishna L. Cemented hemiarthroplasty in traumatic displaced femoral neck fractures and deep vein thrombosis: is there really a link? Singapore Med J. 2016;57:69–72. https://doi.org/10.11622/smedj.2016030.
Juliebo V, Krogseth M, Skovlund E, Engedal K, Wyller TB. Medical treatment predicts mortality after hip fracture. J Gerontol A Biol Sci Med Sci. 2010;65:442–449. https://doi.org/10.1093/gerona/glp199.
Kankanala G, Shivarathre DG, Pidikiti P. A comparative study of the postoperative morbidity and mortality in femoral neck fractures in elderly patients treated with cemented and uncemented Thompson hemiarthroplasty. J Orthop Trauma Rehabil. 2011;15:47–50. https://doi.org/10.1016/j.jotr.2011.04.001.
Keating JF, Grant A, Masson M, Scott NW, Forbes JF. Randomized comparison of reduction and fixation, bipolar hemiarthroplasty, and total hip arthroplasty. Treatment of displaced intracapsular hip fractures in healthy older patients. J Bone Jt Surg Am. 2006;88:249–260. https://doi.org/10.2106/JBJS.E.00215.
Khan RJ, MacDowell A, Crossman P, Datta A, Jallali N, Arch BN, et al. Cemented or uncemented hemiarthroplasty for displaced intracapsular femoral neck fractures. Int Orthop. 2002;26:229–232. https://doi.org/10.1007/s00264-002-0356-2.
Khorami M, Arti H, Aghdam AA. Cemented versus uncemented hemiarthroplasty in patients with displaced femoral neck fractures. Pakistan J Med Sci. 2016;32:44–48. https://doi.org/10.12669/pjms.321.8461.
Kim YH, Suh JS. Low incidence of deep-vein thrombosis after cementless total hip replacement. J Bone Jt Surg Am. 1988;70:878–882.
Langslet E, Frihagen F, Opland V, Madsen JE, Nordsletten L, Figved W. Cemented versus uncemented hemiarthroplasty for displaced femoral neck fractures: 5-year followup of a randomized trial. Clin Orthop Relat Res. 2014;472:1291–1299. https://doi.org/10.1007/s11999-013-3308-9.
Leizorovicz A, Turpie AG, Cohen AT, et al. Epidemiology of venous thromboembolism in Asian patients undergoing major orthopedic surgery without thromboprophylaxis. The SMART study. J Thromb Haemost. 2005;3:28–34. https://doi.org/10.1111/j.1538-7836.2004.01094.x.
Lennox IA, McLauchlan J. Comparing the mortality and morbidity of cemented and uncemented hemiarthroplasties. Injury. 1993;24:185–186.
Li T, Zhuang Q, Weng X, Zhou L, Bian Y. Cemented versus uncemented hemiarthroplasty for femoral neck fractures in elderly patients: a meta-analysis. PLoS One. 2013;8:e68903. https://doi.org/10.1371/journal.pone.0068903.
Lin FF, Chen YF, Chen B, Lin CH, Zheng K. Cemented versus uncemented hemiarthroplasty for displaced femoral neck fractures: A meta-analysis of randomized controlled trails. Medicine (Baltimore). 2019;98(8):e14634. https://doi.org/10.1097/MD.0000000000014634.
Lo WH, Chen WM, Huang CK, Chen TH, Chiu FY, Chen CM. Bateman bipolar hemiarthroplasty for displaced intracapsular femoral neck fractures. Uncemented versus cemented. Clin Orthop Relat Res. 1994;(302):75–82.
Luo X, He S, Li Z, Huang D. Systematic review of cemented versus uncemented hemiarthroplasty for displaced femoral neck fractures in older patients. Arch Orthop Trauma Surg. 2012;132:455–463. https://doi.org/10.1007/s00402-011-1436-9.
Lynch AF, Bourne RB, Rorabeck CH, Rankin RN, Donald A. Deep-vein thrombosis and continuous passive motion after total knee arthroplasty. J Bone Jt Surg Am. 1988;70:11–14.
Marchetti P, Binazzi R, Vaccari V, et al. Long-term results with cementless fitek (or fitmore) cups. J Arthroplasty. 2005;20(6):730–737. https://doi.org/10.1016/j.arth.2004.11.019.
Morris K, Davies H, Wronka K. Implant-related complications following hip hemiarthroplasty: a comparison of modern cemented and uncemented prostheses. Eur J Orthop Surg Traumatol. 2015;25:1161–1164. https://doi.org/10.1007/s00590-015-1671-9.
Ng ZD, Krishna L. Cemented versus cementless hemiarthroplasty for femoral neck fractures in the elderly. J Orthop Surg (Hong Kong). 2014;22:186–189. https://doi.org/10.1177/230949901402200214.
Ng Man Sun S, Gillott E, Bhamra J, Briggs T. Implant use for primary hip and knee arthroplasty: are we getting it right first time? J Arthroplast. 2013;28:908–912. https://doi.org/10.1016/j.arth.2012.11.012.
Ozturkmen Y, Karamehmetoglu M, Caniklioglu M, Ince Y, Azboy I. Cementless hemiarthroplasty for femoral neck fractures in elderly patients. Indian J Orthop. 2008;42:56–60. https://doi.org/10.4103/0019-5413.38582.
Parker MI, Pryor G, Gurusamy K. Cemented versus uncemented hemiarthroplasty for intracapsular hip fractures: A randomised controlled trial in 400 patients. J Bone Jt Surg Br 2010;92:116–22. https://doi.org/10.1302/0301-620x.92b1.22753.
Petersen MB, Jorgensen HL, Hansen K, Duus BR. Factors affecting postoperative mortality of patients with displaced femoral neck fracture. Injury. 2006;37:705–711. https://doi.org/10.1016/j.injury.2006.02.046.
Prashanth YS, Niranjan M. Comparative Study of Surgical Management of Fracture Neck of Femur with Cemented Versus Uncemented Bipolar Hemiarthroplasty. J Clin Diagn Res. 2017;11:RC17–21. https://doi.org/10.7860/JCDR/2017/22598.9454.
Rogmark C, Leonardsson O, Garellick G, Karrholm J. Monoblock hemiarthroplasties for femoral neck fractures—a part of orthopaedic history? Analysis of national registration of hemiarthroplasties 2005-2009. Injury. 2012;43:946–949. https://doi.org/10.1016/j.injury.2011.11.022.
Sadr B, Arden GP. A comparison of the stability of proplast-coated and cemented Thompson prostheses in the treatment of subcapital femoral fractures. Injury. 1977;8:234–237. https://doi.org/10.1016/0020-1383(77)90137-1.
Santini S, Rebeccato A, Bolgan I, Turi G. Hip fractures in elderly patients treated with bipolar hemiarthroplasty: comparison between cemented and cementless implants. J Orthop Traumatol. 2005;6:80–87. https://doi.org/10.1007/s10195-005-0086-5.
Shewale SB, Pandit HG, Latham JM. Hemiarthroplasty: To cement or not to cement? A preliminary report. Hip Int. 2004;14:189–192. https://doi.org/10.5301/hip.2008.976.
Shoda N, Yasunaga H, Horiguchi H, Matsuda S, Ohe K, Kadono Y, et al. Risk factors affecting inhospital mortality after hip fracture: retrospective analysis using the Japanese Diagnosis Procedure Combination Database. BMJ Open. 2012;2. https://doi.org/10.1136/bmjopen-2011-000416.
Sierra RJ, Timperley JA, Gie GA. Contemporary cementing technique and mortality during and after Exeter total hip arthroplasty. J Arthroplast. 2009;24:325–332. https://doi.org/10.1016/j.arth.2008.01.301.
Skyrme AD, Jeer PJ, Berry J, Lewis SG, Compson JP. Intravenous polymethyl methacrylate after cemented hemiarthroplasty of the hip. J Arthroplast. 2001;16:521–523. https://doi.org/10.1054/arth.2001.22399.
Sonne-Holm S, Walter S, Jensen JS. Moore hemi-arthroplasty with and without bone cement in femoral neck fractures. A clinical controlled trial. Acta Orthop Scand. 1982;53:953–956. https://doi.org/10.3109/17453678208992854.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. https://doi.org/10.1007/s10654-010-9491-z.
Talsnes O, Hjelmstedt F, Dahl OE, Pripp AH, Reikeras O. Biochemical lung, liver and kidney markers and early death among elderly following hip fracture. Arch Orthop Trauma Surg. 2012;132:1753–1758. https://doi.org/10.1007/s00402-012-1611-7.
Talsnes O, Hjelmstedt F, Pripp AH, Reikeras O, Dahl OE. No difference in mortality between cemented and uncemented hemiprosthesis for elderly patients with cervical hip fracture. A prospective randomized study on 334 patients over 75 years. Arch Orthop Trauma Surg. 2013;133:805–809. https://doi.org/10.1007/s00402-013-1726-5.
Taylor F, Wright M, Zhu M. Hemiarthroplasty of the hip with and without cement: a randomized clinical trial. J Bone Jt Surg Am. 2012;94:577–583. https://doi.org/10.2106/JBJS.K.00006.
Tripuraneni KR, Carothers JT, Junick DW, Archibeck MJ. Cost comparison of cementless versus cemented hemiarthroplasty for displaced femoral neck fractures. Orthopedics. 2012;35:e1461–1464.
Veldman HD, Heyligers IC, Grimm B, Boymans TA. Cemented versus cementless hemiarthroplasty for a displaced fracture of the femoral neck: a systematic review and meta-analysis of current generation hip stems. Bone Jt J. 2017;99-b:421–431. https://doi.org/10.1302/0301-620x.99b4.Bjj-2016-0758.R1.
Viberg B, Overgaard S, Lauritsen J, Ovesen O. Lower reoperation rate for cemented hemiarthroplasty than for uncemented hemiarthroplasty and internal fixation following femoral neck fracture: 12- to 19-year follow-up of patients aged 75 years or more. Acta Orthop. 2013;84:254–259. https://doi.org/10.3109/17453674.2013.792033.
Vidovic D, Punda M, Darabos N, Bekavac-Beslin M, Bakota B, Matejcic A. Regional bone loss following femoral neck fracture: A comparison between cemented and cementless hemiarthroplasty. Injury. 2015;46 Suppl 6:S52–S56. https://doi.org/10.1016/j.injury.2015.10.069.
Wilson D, Cooke EA, McNally MA, Wilson HK, Yeates A, Mollan RA. Altered venous function and deep venous thrombosis following proximal femoral fracture. Injury. 2002;33:33–39.
Yli-Kyyny T, Ojanpera J, Venesmaa P, , et al. Perioperative complications after cemented or uncemented hemiarthroplasty in hip fracture patients. Scand J Surg. 2013;102:124–128. https://doi.org/10.1177/1457496913482249.
Yli-Kyyny T, Sund R, Heinanen M, Venesmaa P, Kroger H. Cemented or uncemented hemiarthroplasty for the treatment of femoral neck fractures? Acta Orthop. 2014;85:49–53. https://doi.org/10.3109/17453674.2013.878827.
Yurdakul E, Karaaslan F, Korkmaz M, Duygulu F, Baktir A. Is cemented bipolar hemiarthroplasty a safe treatment for femoral neck fracture in elderly patients? Clin Interv Aging. 2015;10:1063–1067. https://doi.org/10.2147/cia.S85039.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Mohamed A. Imam, Mohamed S. A. Shehata, Mahmoud Morsi, Muhammad Shawqi, Ahmed Elsehili, Paul Trikha, Lukas Ernstbrunner, Ashwin Unnithan, Arshad Khaleel, Puneet Monga, Ali Narvani, and Asser Sallam, MD, PhD, declare that they have no conflicts of interest.
Human/Animal Rights
N/A
Informed Consent
N/A
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Additional information
Level of Evidence: Level IV: Systematic review of Level I–IV studies
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Imam, M., Shehata, M., Morsi, M. et al. The Effect of Type of Femoral Component Fixation on Mortality and Morbidity after Hip Hemiarthroplasty: A Systematic Review and Meta-Analysis. HSS Jrnl 16, 222–232 (2020). https://doi.org/10.1007/s11420-020-09769-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11420-020-09769-1