Abstract
Intermittent parathyroid hormone administration can enhance fracture healing in an animal model. Despite the success of exogenous parathyroid hormone on fracture healing and spine fusion, few studies have examined the role of parathyroid hormone on cartilage formation. We determined the effects of intermittent parathyroid hormone on cartilage formation in a rabbit microfracture model of cartilage regeneration. Twelve rabbits were divided into three equal groups: (1) microfracture alone, (2) microfracture + parathyroid hormone daily for 7 days, and (3) microfracture + parathyroid hormone for 28 days. Nonoperated contralateral knees were used as controls. The animals were sacrificed at 3 months and gross and histologic analysis was performed. The microfracture alone group demonstrated the most healing on gross and histologic analysis. Treatment with either 1 or 4 weeks of parathyroid hormone inhibited cartilage formation. Although discouraging from a cartilage repair point of view, this study suggests that the role parathyroid hormone administration has in clinical fracture healing must be examined carefully. Although parathyroid hormone is beneficial to promote healing in spine fusion and midshaft fractures, its deleterious effects on cartilage formation suggests that it may have adverse effects on the outcomes of periarticular fractures such as tibial plateau injuries that require cartilage healing for a successful clinical outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Full-thickness cartilage defects remain one of the most common, yet difficult challenges faced by orthopedic surgeons today. Curl et al. [1] reported in over 31,000 knee arthroscopies that 63% of patients had a grade III or IV [2, 3] articular cartilage lesion. These lesions demonstrate limited healing potential, with full-thickness cartilage lesions healing with both types I and II cartilage, and lower than normal concentrations of proteoglycans [4]. The formation of structurally inferior fibrocartilage leads to premature irregularities of the joint surface over time, meniscal tears, and, eventually, degenerative arthritis.
Microfracture was developed by Steadman et al. [5] as a novel technique to treat full-thickness cartilage lesions. The technique of microfracture uses an awl to penetrate the subchondral plate, which leads to migration of mesenchymal stem cells into the cartilage defect and reconstitution of the injured area with a fibrocartilage repair with varying amounts of types I, II, and III collagen content [6]. Microfracture reportedly results in 70% to 90% of patients having short-term improvement in function [5–9]. However, a small but substantial percentage has only moderate to poor fill and worse subjective and objective clinical outcomes [8].
Despite the clinical success of microfracture in healing small (<2 cm) cartilage defects in the knee [5, 10, 11], there have been many attempts to improve the quality and fill of these lesions. Penetration of the subchondral bone allows for mesenchymal stem cells to enter into a localized defect and produce fibrocartilage. Current surgical techniques typically allow for complete fill in 54%, partial fill in 29%, and poor fill in 17% [5]. Not surprisingly, there is a correlation between amount of fill and clinical outcome. As the results of microfracture have been variable, there have been many attempts to increase the likelihood of complete lesion fill with hyaline cartilage. Previous animal studies have utilized bone morphogenetic proteins (BMPs), transforming growth factor-β, and insulin-like growth factor-1 to stimulate cartilage healing with promising results [12–14]. In a recent study, Morgan et al. [12] found BMP-7, which induces cartilage formation, worked synergistically with microfracture to heal full-thickness cartilage defects in a rabbit model. Despite the early success of these studies in animal models, no recombinant growth factors have been successfully utilized in clinical trials to our knowledge.
The role of exogenous parathyroid hormone (PTH) in fracture healing continues to be elucidated. Alkhiary et al. [15] originally reported that intermittent administration of PTH enhanced fracture healing in a rat fracture model. The authors found that treatment with 80 μg/kg PTH increased callus area and strength, with an increase in the amount of new bone formed with histologic analysis [15]. Numerous subsequent studies have found similar results [16–18]. Recent studies have found that there is increased chondrogenesis with exogenous administration of PTH. Manabe et al. [17] reported increased expression of SOX9 and procollagen α-1 and increased chondrocyte proliferation in animals receiving intermittent PTH. SOX9 is a high-mobility group domain transcription factor expressed in chondrocytes during cartilage formation and is essential for cartilage formation in SOX9−/− transgenic mice [19]. Expression of SOX9 is decreased in cartilage defects that failed to heal, highlighting the importance of this transcription factor in healing of cartilage lesions. Similarly, Lane et al. [20] reported increased expression of Wnt 4, 5a, 5b, and 10b in animals treated with intermittent PTH to stimulate fracture healing. Taken together, these studies suggest that exogenous PTH stimulates fracture healing by increasing the activity of genes such as SOX9 and the Wnt family of proteins to increase chondrogenesis and promote cartilage formation.
The purpose of this study was to evaluate the effects of exogenous intermittent PTH administration on (1) the gross and (2) the microscopic cartilage healing in a rabbit microfracture model. Based on the improved chondrogenesis seen with the use of PTH in a fracture healing model, we hypothesized that the same mechanisms would stimulate improved cartilage formation in a microfracture setting We specifically aimed to measure the effect that exogenous, intermittent PTH administration has following 1- and 4-week treatment periods on the gross and histologic appearance of cartilage defects treated by microfracture compared to animals treated with microfracture alone.
Materials and methods
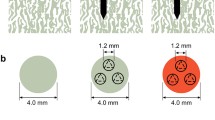
Female adolescent New Zealand White rabbits (n = 12), weighing from 3.1 to 3.5 kg, were utilized for this study. The animals were divided into three groups: microfracture alone, microfracture + 10 μg/kg human recombinant (1–34) PTH for 7 days, and microfracture + 10 μg/kg recombinant (1–34) PTH for 28 days (Fig. 1). The control group received a saline injection for 7 days. The PTH was administered subcutaneously on a daily basis commencing on the day of surgery and continued until the specified day. Standard anteroposterior and lateral radiographs of the affected knee were obtained at the time of sacrifice to identify any osseous changes. The research protocol was approved by the animal ethics committee of the Hospital for Special Surgery and animal care and experimental procedures were approved by the animal institutional review board.
a–c Diagrams illustrate the experimental design for the PTH treatment of 6-mm-diameter full-thickness defects of articular cartilage. a Saline was administered as a control, b 10 μg/kg PTH was administered daily for 1 week, or c 10 μg/kg PTH was administered daily for 4 weeks. The animals were sacrificed at 12 weeks (triangle). The distal portion of the femur was removed and fixed as described in “Materials and methods” section
The rabbit microfracture model has been previously described [18]. Animals were anesthetized with sodium pentobarbital and the left leg was prepped and draped. Sterile procedure was used for every animal. A midline incision was made over the knee and a medial parapatellar arthrotomy was performed to expose the knee. The patella was reflected laterally to expose the femoral trochlea. A 6-mm full-thickness defect was made on the weight-bearing surface of the trochlea using a curette. Microfracture was performed with an 18-gauge needle. The microfracture holes punctured the subchondral plate and blood was seen to exit each of the holes. The wounds were closed and the animal was allowed to bear weight immediately. Pain medication was administered as needed after the procedure.
At the time of sacrifice, the animals were given a lethal dose of sodium pentobarbital. At the sacrifice, the soft tissue was removed from the distal femur, and the femur was sectioned approximately 1 cm above the joint. The patella and proximal tibia were removed from the specimen as well. The contralateral femur was removed as a nonoperated control. We scored the gross appearance of the lesion based on the fill of the lesion (1 = no fill; 2 = minimal fill; 3 = near-complete fill; 4 = complete fill), quality of the tissue within the lesion (1 = no tissue; 2 = soft tissue within the lesion; 3 = normal appearing tissue within the lesion), and surrounding changes (1 = substantial degenerative cartilage changes surrounding the lesion with loss of cartilage down to bone; 2 = slight changes in the surrounding cartilage without loss of cartilage to bone; 3 = no changes). This allowed for a calculation of a gross score with a maximum of 10 points.
Following gross examination, the distal femurs were fixed in 4% paraformaldehyde at room temperature and then transferred to 10% ethylenediaminetetraacetic acid for 3 weeks for decalcification. The specimens were then embedded in paraffin for subsequent histologic analysis. Five-micrometer transverse sections were taken through the center of the defect and stained with hematoxylin and eosin or alcian blue. For semiquantitative analysis of cartilage restoration, we (BF, ZD) examined the sections in a blinded manner and scored according to the grading scale of Pineda et al. [11] with modifications as described by Mizuta et al. [13] (Table 1). The maximum score with this scale is 14, indicating complete fill with reconstitution of the osteochondral junction, normal staining of the matrix, and normal cell morphology.
Data are presented as the mean ± standard deviation. Differences between groups in both the gross and histologic score were evaluated with a one-way analysis of variance. A value of p < 0.05 was defined as statistically significant.
Results
At the time of sacrifice, there were no complications due to PTH administration, and all animals appeared to tolerate the treatment well. One animal developed ossification around the knee and a flexion contracture during the last week of the study. This animal was treated with a microfracture alone, and therefore, this was not a complication of PTH administration. Radiographs of this animal showed soft tissue calcification surrounding the knee. No other animals had any important radiographic changes at the time of sacrifice.
Administration of PTH inhibited or delayed the regeneration of cartilage in the microfracture sites compared to the untreated animals. Grossly, all animals in the microfracture alone group appeared to have completely healed their defects with normal-appearing cartilage in three of four animals. One animal had cartilage that felt softer than the other animals. The overall gross score was 9.1 ± 0.8. In the 1-week PTH group, there was minimal or no new cartilage present and the defects remained present in all the animals. One animal had erosive changes surrounding the lesion. The overall gross score was 2.3 ± 1.4 (p < 0.001 versus control). In the 4-week PTH group, there was minimal cartilage formed in two of the four animals although the cartilage felt soft. One animal in this group had substantial erosive changes surrounding the lesion as well. The overall gross score was 3.6 ± 2.5 (p < 0.001 versus control; p = NS versus 1-week PTH; Fig. 2).
a–c Photographs show the gross appearance of representative specimens at the time of sacrifice. a In the microfracture alone specimen, there is reconstitution of the lesion with near-complete fill and normal-appearing cartilage. b In the microfracture + 1-week PTH specimen, the lesion is still clearly evident with minimal fill. In addition, the surrounding cartilage appears to have thinned and undergone erosive changes. c In the microfracture + 4-week PTH specimen, similar to the 1-week treatment group, the lesion remains evident with minimal fill within the lesion. There appears to be some mild erosive as well
Histologic analysis confirmed the findings of the gross analysis further suggesting that PTH administration inhibited cartilage regeneration in the microfracture sites. There was near-complete fill of the defect in the microfracture alone group, although there were fewer cells and reduced matrix staining within the microfracture lesion compared to control contralateral femurs (Fig. 3a). In the 1-week PTH group, there was no fill of the lesion and there appeared to be thinning of the surrounding cartilage in all the samples (Fig. 3b). Similar results were found in the 4-week PTH group, with minimal fill of the defect and thinning of the surrounding cartilage and decreased matrix staining (Fig. 3c). The average histologic score in the microfracture alone group was 10.3 ± 3.4, compared to 2.7 ± 2.5 in the 1-week PTH group (p < 0.001 versus microfracture alone) and 3.4 ± 2.5 in the 4-week PTH group (p < 0.001 versus microfracture alone; Fig. 4).
a–c Photomicrographs show the histologic appearance of representative specimens at the time of sacrifice (stain, hematoxylin and eosin, alcian blue; original magnification, ×10). a In the microfracture alone specimen, there is reconstitution of the lesion with near-complete fill, although there are fewer than normal cells and the matrix staining is less than normal. b In the microfracture + 1-week PTH specimen, the lesion is still clearly evident with minimal fill. In addition, the surrounding cartilage appears to have thinned with decreased matrix staining. c In the microfracture + 4-weeks PTH specimen, no cartilage formation is evident within the lesion, and the surrounding cartilage appears to be thinned as well
A graph shows the histologic score as described by Pineda et al. [11] for the three treatment groups. †p < 0.001 versus control animals. MFx microfracture
Discussion
Intermittent PTH administration can enhance fracture healing in an animal model. Despite the success of exogenous PTH on fracture healing and spine fusion, there have been few studies examining the role of PTH on cartilage healing. We investigated the role of intermittent administration of exogenous recombinant PTH on healing of a microfracture model. Based on the improved chondrogenesis seen with the use of PTH in a fracture healing model, we hypothesized that the same mechanisms would stimulate improved cartilage healing in a microfracture setting. Contrary to our hypothesis, we found that intermittent PTH given for 1 or 4 weeks was effective at inhibiting cartilage production in this model. Although disappointing from a cartilage repair standpoint, our results are interesting as it increases our understanding of the role of PTH in bone and cartilage formation.
There were several weaknesses to this study. First, since we designed a preliminary study, we used no alternate doses or administration time courses. A dose of 10 µg/kg was chosen based on its success in previous studies that it found successfully improved bone formation via chondrogenesis [17, 18]. Likewise, a 1- and 4-week time course of PTH administration was chosen based on its success in previous studies [17, 18]. It is possible that a shorter period of time (i.e., 2–5 days) would be sufficient to stimulate cartilage formation without leading to inhibition of osteochondral healing. Second, the underlying mechanism of inhibition of cartilage formation with intermittent PTH is not clearly understood. With continuous PTH administration, there is downregulation of the PTH/PTH-related protein (PTHrP) receptors, but it is unknown if intermittent PTH functions by the same mechanism to inhibit cartilage repair. Clearly, these factors should be clarified in subsequent studies.
Based on the promising results with other growth factors and the enhanced chondrogenesis seen using recombinant intermittent PTH in a fracture healing model, we presumed that administration of 10 µg/kg intermittent PTH would enhance cartilage formation in a microfracture model. However, we found that intermittent PTH actually nearly completely inhibited the formation of new cartilage within the defect in our model. With 1 week of PTH, there was no cartilage formed within the defect, a finding that was clearly evident on both gross and histologic examination. The findings were similar in animals treated for 4 weeks of intermittent PTH. On gross examination, there was minimal or no fill in almost all the animals treated with intermittent PTH, suggesting an inhibition of chondrocyte proliferation. A smaller proportion of the animals also demonstrated adjacent cartilage breakdown after treatment with PTH. Interestingly, there appeared to be decreased staining of the surrounding cartilage matrix in those animals treated with PTH, suggesting an alteration of collagen or proteoglycan concentration in these animals. These results clearly demonstrate that intermittent PTH administration is not an effective means to improve the ability of microfracture to heal chondral defects.
Two previous studies utilizing continuous PTH administration in a chondral defect model have demonstrated results similar to ours. Nozaka et al. [21] reported that PTH, when given continuously via an osmotic pump into the knee, completely inhibited chondrogenesis. The authors found that, although proliferation of the chondral precursors was normal, PTH administration effectively inhibited chondrogenic differentiation. In a subsequent study, the authors found that continuous PTH administration for 4 weeks was able to irreversibly block expression of PTH/PTHrP receptor, suggesting cells that migrate into the chondral defect maintain their chondrogenic potential for no more than 2 weeks [22]. Our data suggest that even intermittent PTH administration results in a similar outcome in this model. This is surprising as it is traditionally thought that continuous and intermittent PTH function in separate ways in a fracture healing model. These results demonstrate that it is vital to more completely understand the mechanisms that govern the outcome of PTH administration in osteochondral injury models. This is particularly true in cases of fractures that enter the joint. Although it may be alluring to use PTH to improve fracture healing in these difficult to treat fracture models, it is possible that the PTH would have a deleterious effects on the cartilage injury and result in a much poorer overall outcome.
We found that intermittent PTH did not improve healing of full-thickness chondral defects in a rabbit microfracture model and in fact inhibited cartilage formation within the defect. Although these results did not support our hypothesis, they do highlight the importance of understanding the molecular mechanisms that govern the relationship between PTH and chondrogenesis. Future studies focusing on the molecular mechanisms of PTH-mediated chondrogenesis and the time course of gene expression may help elucidate why PTH is effective in stimulating cartilage formation in a fracture healing model yet is unable to stimulate cartilage in a full-thickness chondral injury model.
References
Curl WW, Krome J, Gordon ES, Rushing J, et al. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997; 13: 456–460.
Outerbridge RE. The etiology of chondromalacia patellae. J. Bone Joint Surg. Br. 1961; 43B: 752–757.
Outerbridge RE, Dunlop JA, The etiology of chondromalacia patellae. Clin. Orthop. Rel. Res. 1975; 110: 177–196.
Gill TJ, The treatment of articular cartilage defects using microfracture and debridement. Am. J. Knee Surg. 2000; 13: 33–40.
Steadman JR, Miller BS, Karas SG, et al. The microfracture technique in the treatment of full-thickness chondral lesions of the knee in National Football League players. J. Knee Surg. 2003; 16: 83–86.
Williams RJ, 3rd, Harnly HW. Microfracture: indications, technique, and results. Instr. Course Lect. 2007; 56: 419–428
Kreuz PC, Steinwachs MR, Erggelet C, et al. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthr. Cartil. 2006; 14: 1119–1125.
Mithoefer K, Williams RJ, 3rd, Warren RF, et al. The microfracture technique for the treatment of articular cartilage lesions in the knee. A prospective cohort study. J. Bone Joint Surg. Am. 2005; 87: 1911–1920.
Gobbi A, Nunag P, Malinowski K. Treatment of full thickness chondral lesions of the knee with microfracture in a group of athletes. Knee Surg. Sports Traumatol. Arthrosc. 2005; 13: 213–221.
Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthr. Cartil. 2002; 10: 432–463.
Pineda S, Pollack A, Stevenson S, et al. A semiquantitative scale for histologic grading of articular cartilage repair. Acta Anat. (Basel). 1992; 143: 335–340.
Morgan EF, Mason ZD, Bishop G, et al. Combined effects of recombinant BMP-7 (rhBMP-7) and parathyroid hormone (1–34) in metaphyseal bone healing. Bone. 2008; 43: 1031–1038.
Mizuta H, Kudo S, Nakamura E, et al. Expression of the PTH/PTHrP receptor in chondrogenic cells during the repair of full thickness defects of articular cartilage. Osteoarthr. Cartil. 2006; 14: 944–952.
Bi W, Deng JM, Zhang Z, et al. Sox9 is required for cartilage formation. Nat. Genet. 1999; 22: 85–89.
Alkhiary YM, Gerstenfeld LC, Krall E, et al. Enhancement of experimental fracture-healing by systemic administration of recombinant human parathyroid hormone (PTH 1–34). J. Bone Joint Surg. Am. 2005; 7: 731–741.
Hashimoto T, Shigetomi M, Ohno T, et al. Sequential treatment with intermittent low-dose human parathyroid hormone (1–34) and bisphosphonate enhances large-size skeletal reconstruction by vascularized bone transplantation. Calcif. Tissue Int. 2007; 81: 232–239.
Manabe T, Mori S, Mashiba T, et al. Human parathyroid hormone (1–34) accelerates natural fracture healing process in the femoral osteotomy model of cynomolgus monkeys. Bone. 2007; 40: 1475–1482.
Kuo AC, Rodrigo JJ, Reddy AH, et al. Microfracture and bone morphogenetic protein 7 (BMP-7) synergistically stimulate articular cartilage repair. Osteoarthr. Cartil. 2006; 14: 1126–1135.
Nakajima A, Shimoji N, Shiomi K, et al. Mechanisms for the enhancement of fracture healing in rats treated with intermittent low-dose human parathyroid hormone (1–34). J. Bone Miner. Res. 2002; 17: 2038–2047.
Lane JM, Gardner MJ, Lin JT, et al. The aging spine: new technologies and therapeutics for the osteoporotic spine. Eur Spine J. 2003; 12(Suppl 2): S147–S154.
Nozaka K, Miyakoshi N, Kasukawa Y, et al. Intermittent administration of human parathyroid hormone enhances bone formation and union at the site of cancellous bone osteotomy in normal and ovariectomized rats. Bone. 2008; 42: 90–97.
Kudo S, Mizuta H, Otsuka Y, et al. Inhibition of chondrogenesis by parathyroid hormone in vivo during repair of full-thickness defects of articular cartilage. J. Bone Miner. Res. 2000; 15: 253–260.
Acknowledgments
The authors would like to thank Eli Lilly and Co. (Indianapolis, IN) for supplying the PTH.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Each author certifies that he or she has no commercial associations (e.g., consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Feeley, B.T., Doty, S.B., Devcic, Z. et al. Deleterious Effects of Intermittent Recombinant Parathyroid Hormone on Cartilage Formation in a Rabbit Microfracture Model: a Preliminary Study. HSS Jrnl 6, 79–84 (2010). https://doi.org/10.1007/s11420-009-9134-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11420-009-9134-7